Unconventional Electrophiles
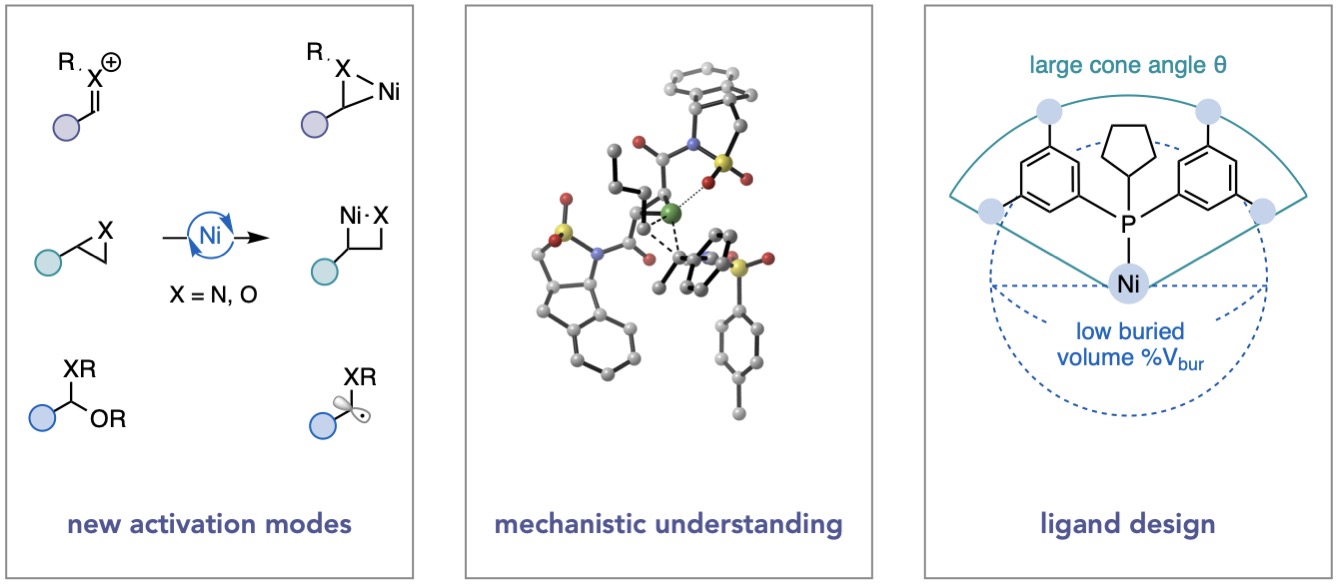
One of our group’s contributions has been to design methods for Ni-catalyzed cross coupling that enable direct access to important bioactive motifs from unconventional electrophile partners. For example, we have introduced transformations that leverage the reactivity of iminium ions, oxocarbenium ions, epoxides, and aziridines with Ni. These electrophiles and their precursors are abundant, stable, and versatile intermediates in synthetic organic chemistry. We have shown that engaging these electrophiles with Ni intersects the modularity of cross coupling in terms of its scope and functional group tolerance, while directing reactivity toward classes of products that are not readily available by alternative means. Recent work in the group has centered on developing both reductive and photo-assisted cross-electrophile couplings. Examples of this work include a stereoconvergent cross-coupling of epoxides and aryl iodides, a linear selective cross-coupling of alkyl aziridines and aryl iodides, and a three-component C–C bond-forming reductive amination from in situ generated iminium ions. Ongoing research is focused on elucidating the various pathways by which C(sp3)–C bond formation occurs, expanding the scope of coupling partners for these reactions, and developing ligand platforms that can engender high chemo- and stereoselectivity.
Mechanistic Studies
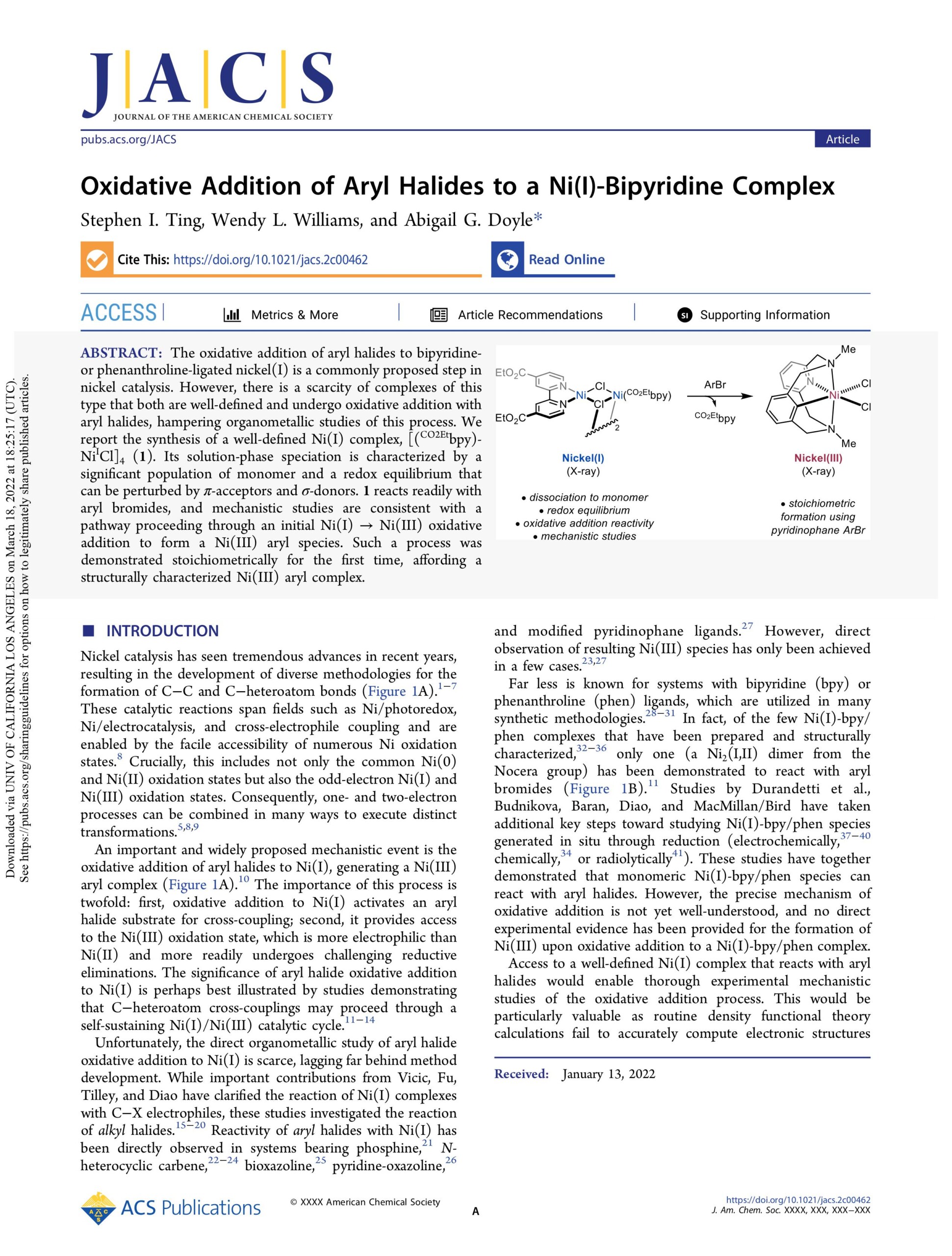
Our group has also undertaken fundamental organometallic studies of catalytically relevant systems. Through these studies, we are able to draw conclusions that inform methodological development, ligand design, and other mechanistic studies. Using high-throughput experimentation (HTE) and data science, we were able to identify a steric descriptor, minimum percent buried volume (%Vbur(min)), that univariately classifies monophosphine ligands into catalytically successful or low-yielding regimes. Using an organometallic model system, we were able to rationalize the observed reactivity by showing that only monophosphines below %Vbur(min) ≈ 32% are capable of forming bisligated Ni species. Another study from our group examined bipyridine-ligated nickel complexes in the +1 oxidation state. Such complexes are frequently invoked in catalytic cycles for Ni, but rapid redox equilibria obfuscate well-defined systems. In this study, we were able to identify such a well-defined system and demonstrate that aryl halides can undergo oxidative addition to bipyridine-ligated nickel(I) complexes—the first clear evidence for a commonly proposed elementary step. The Doyle lab is currently investigating ligand descriptors that describe catalytic reactivity, the structure and reactivity of well-defined nickel(I) complexes, and catalytic enhancement provided by remote steric bulk in phosphine ligands.
Ligand Design
While recent advances in the field of Pd-catalyzed cross coupling have been driven largely by ligand and precatalyst design, related studies for Ni have received less attention. The success of our efforts in methodology development has relied on the development of new ligand and precatalyst platforms tailored specifically for Ni. One such area has focused on the development of electron-deficient olefins (EDO) as ligands in aziridine cross-coupling reactions. We discovered a class of ligands, including Fro-DO, that facilitate the generation of quaternary carbon centers via C(sp3)–C(sp3) reductive elimination. Our lab has also developed a novel class of monodentate phosphine ligands containing aryl groups with substitution at the 3,5-positions. These ligands (named TyrannoPhos and TriceraPhos) confer high activity upon Ni catalysts for challenging Suzuki cross-coupling reactions. In both cases, we have used experimental, computational, and data science experiments to elucidate the structural and mechanistic underpinnings of the ligands’ unique reactivity. We are continuing to explore the advancement, mechanistic elucidation, and application of these ligand classes across a range of Ni-catalyzed coupling reactions.