Photocatalysis
A major research focus of the Doyle lab is using photocatalysis to achieve mild cross electrophile coupling, C–H functionalization, and C–O bond activation. Within this area of research, we have developed numerous reactions utilizing catalytic platforms in Ni/photoredox catalysis, oxidative radical-polar crossover (ORPC), and phosphoranyl radical chemistry.
Ni/Photoredox Catalysis
Over the past decade, our lab has built a program in nickel-catalyzed C(sp3)–C cross-coupling. Given nickel’s proclivity to both accept and generate reactive radical species, we questioned whether carbon-centered radicals generated by visible light photoredox catalysis could be intercepted by nickel to effect cross coupling. Our group, alongside the MacMillan and Molander groups, first demonstrated that the merger of nickel and photoredox catalysis is possible and can enable otherwise challenging bond-forming reactions. Since the emergence of Ni/photoredox catalysis, our group has developed a broad array of C–C bond forming methodologies, expanding the scope of coupling partners employed in traditional Ni-catalyzed cross-couplings. Additionally, our group has a longstanding interest in asymmetric catalysis and has demonstrated the success of chiral ligands in promoting stereoselectivity in a variety of Ni/photoredox cross-coupling methodologies.

Oxidative Radical Polar Crossover
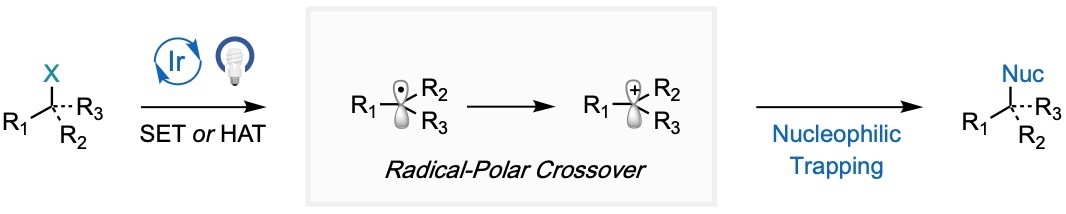
An evolving subset of photocatalysis centered around overcoming challenges with radical and polar chemistry involves merging the two concepts into what is commonly known as radical-polar crossover (RPC). The general strategy involves forming a radical intermediate from the interaction with an excited-state photocatalyst either through a single electron transfer (SET) or hydrogen atom transfer (HAT) and subjecting the intermediate to a single electron oxidation to generate the corresponding carbocation. Hence, this allows for a switch into the ionic reaction space, where two-electron chemistry between the carbocation and a nucleophile can provide novel products. Notably, RPC offers an inherently mild strategy that is compatible with a range of nucleophiles to rapidly incorporate molecular complexity that could not be constructed with either of the individual concepts alone. Our lab has employed this tactic in the nucleophilic fluorination of redox-active esters to access a range of both 18F and 19F-compounds that would be challenging to prepare using conventional nucleophilic and electrophilic methods. More recently, our lab disclosed a radical redox annulation of redox-active radical precursors containing tethered nucleophiles and alkenes to provide direct access to a range of saturated heterocycles.
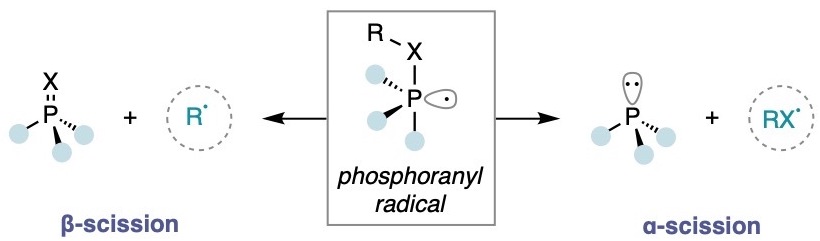
Phosphoranyl Radicals
Our lab also has an interest in exploiting phosphoranyl radical reactivity to activate strong bonds under the mild conditions conferred by photoredox catalysis. Using the β-scission pathway of a phosphine-photoredox dual system, we have developed a direct route to the deoxygenation of benzylic alcohols and carboxylic acids, as well as the hydroacylation of olefins with carboxylic acids. More recently, we have explored the α-scission reactivity pathway of phosphoranyl radicals and reported on a catalytic strategy for the N-H activation of sulfonamides.