Nucleophilic Fluorination
Fluorinated scaffolds are featured in an array of medicines, agrochemicals, and materials due to the unique chemical properties conferred by fluorine substitution. However, the limited availability of synthetic methods to generate carbon-fluorine (C–F) bonds poses a significant challenge to the discovery and production of fluorinated compounds. An ongoing program of research in the Doyle laboratory is focused on the development of catalytic strategies and novel reagents to address limitations in nucleophilic fluorination.
Catalytic Methods
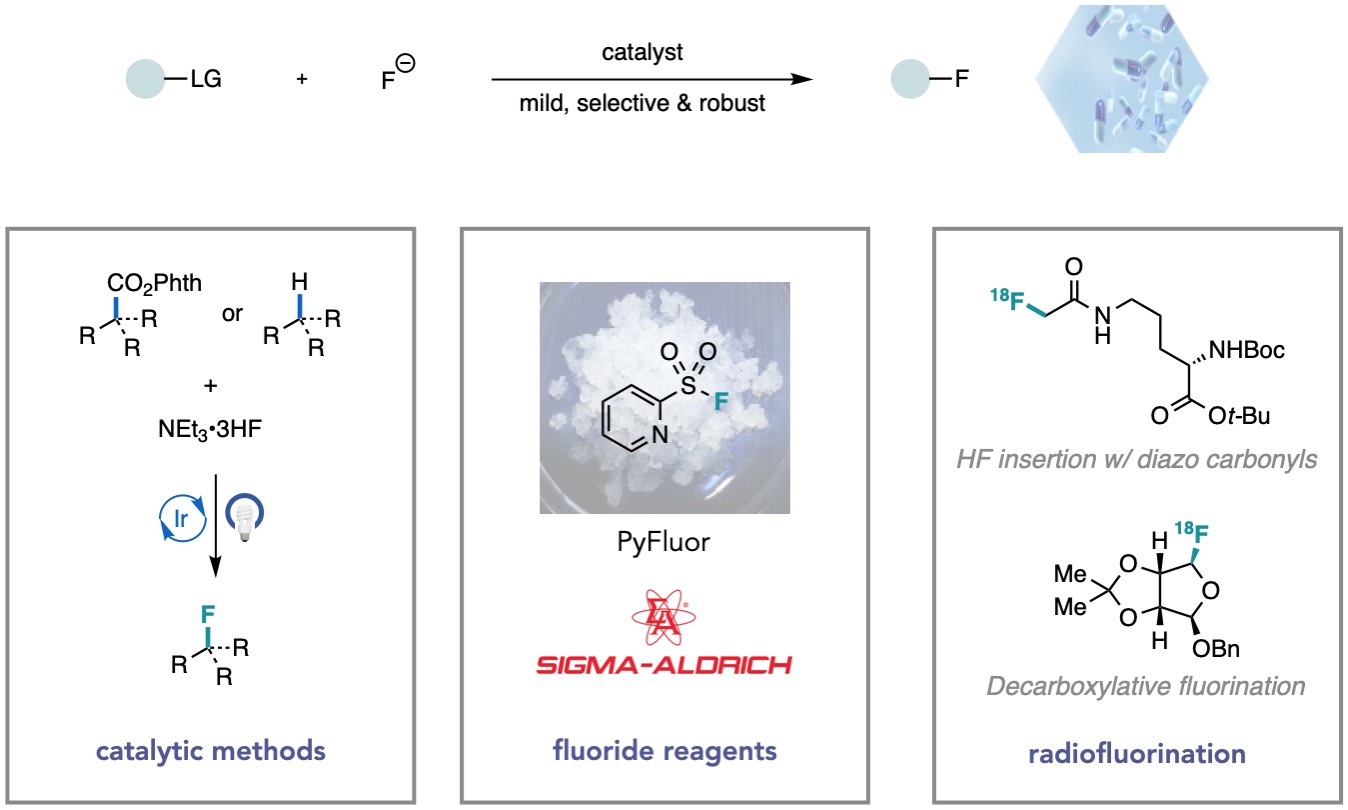
Over the years our group has contributed to the development of various catalytic nucleophilic fluorination methods, including transition-metal catalysis, organocatalysis, and photoredox catalysis. The broader goal of our current research is to enable these transformations under milder conditions to generate value-added building blocks starting from feedstock materials. Our laboratory developed the first asymmetric catalytic aliphatic fluorination reactions using nucleophilic fluoride reagents. For example, we disclosed (salen)Co-catalyzed enantioselective fluoride ring-opening reactions of epoxides and aziridines, as well as Pd-catalyzed asymmetric allylic fluorination of allylic chlorides. Recently, we have utilized visible light photoredox catalysis to develop redox-neutral methods for SN1-type nucleophilic fluorination. Ongoing research studies in our laboratory include deoxy- and deaminative fluorination, C(sp3)-H functionalization, and asymmetric fluorination.
Reagent Development
The most abundant and inexpensive fluorine sources, metal fluoride salts, typically suffer from low solubility, high hygroscopicity, and strong Brønsted basicity, rendering them recalcitrant reagents in chemical synthesis. To overcome the reluctant reactivity inherent to fluoride, our group has developed several novel strategies to accomplish nucleophilic fluorination. One example is PyFluor, a stable and low-cost deoxyfluorination reagent that fluorinates a broad range of alcohols without substantial formation of elimination side products. We have also developed strategies that use benzoyl fluoride as a latent source of fluoride anion which, under Lewis base catalysis, can serve as a potent nucleophilic fluorinating reagent.
Radiofluorination
One roadblock for PET imaging is the chemical “tagging” of small-molecule tracers with a radioisotope, most commonly 18F, to mediate the imaging studies in vivo. A goal of our lab is to develop mild, general, robust, and rapid methods for late-stage incorporation of 18F into small molecules. To this end, we have translated our methods for Co-catalyzed fluoride ring opening of epoxides, Cu-catalyzed insertion of HF into a-diazo carbonyl derivatives, and most recently, decarboxylative fluorination of N-hydroxyphthalimide esters into mild and robust protocols for radiofluorination. Furthermore, building upon our discovery of the deoxyfluorinating reagent PyFluor, we have identified the first no-carrier-added deoxy-radiofluorination.






