Publications
- All
- 2026
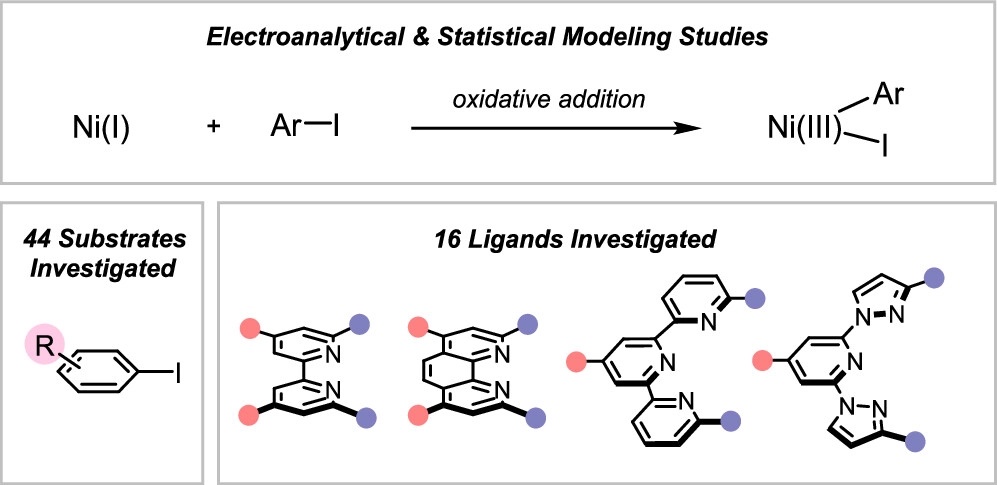
- Ni Cross-Coupling
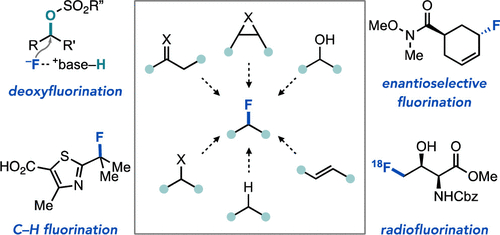
- Nucleophilic Fluorination
- Photocatalysis
- Machine Learning & Data Science
- 2025
- 2024
- 2023
- 2022
- 2021
- 2020
- 2019
- 2018
- 2017
- 2016
- 2015
- 2014
- 2013
- 2012
- 2011
- 2010
107. “Transferable enantioselectivity models from sparse data” Gallarati, S.; Bucci, E. M.; Doyle, A. G.; Sigman, M. S. Nature, Accelerated Preview. [DOI: 10.1038/s41586-026-10239-7] Link PDF

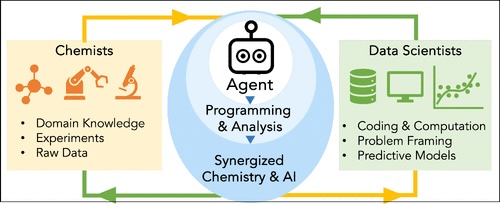
106. “Synergizing Chemical and AI Communities for Advancing Laboratories of the Future” Oh, S.; Fang, X.; Lin, I.-H.; Dee, P.; Dunham, C. S.; Copp, S. M.; Doyle, A. G.; de Alaniz, J. R.; Gu, M. ACS Cent. Sci., ASAP. [DOI: 10.1021/acscentsci.5c01994] Link PDF
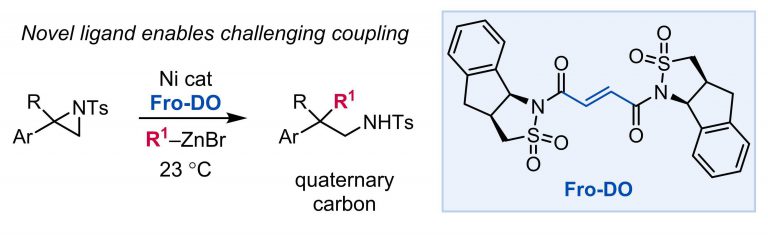
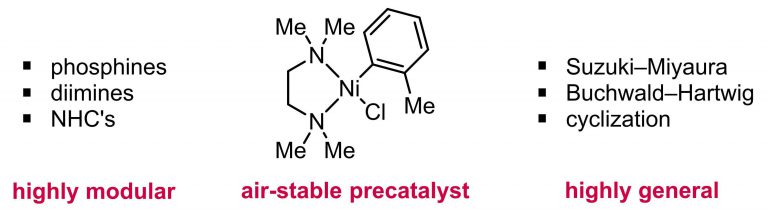
105. “Development, Application, and Mechanistic Interrogation of a Dual Ni Catalysis Approach to Photoredox-Based C(sp3)–C(sp3) Cross-Coupling” Bucci, E. M.; Perea, M. A.; Lalisse, R. F.; Mukherjee, P.; Raab, T. J.; Kannadi Valloli, L.; Min, D. S.; Bird, M. J.; Gutierrez, O.; Doyle, A. G. J. Am. Chem. Soc., 2025, 147, 42311–42329. [DOI: 10.1021/jacs.5c11247] Link PDF

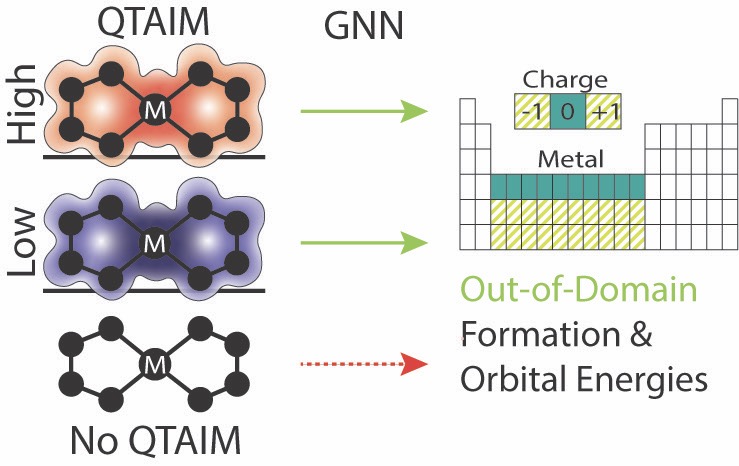
104. “Multi-level QTAIM-Enriched Graph Neural Networks for Resolving Properties of Transition Metal Complexes” Gee, W.; Doyle, A. G.; Vargas, S.; Alexandrova, A. Digital Discovery, 2025, 4, 3378–3388. [DOI:10.1039/D5DD00220F] Link PDF
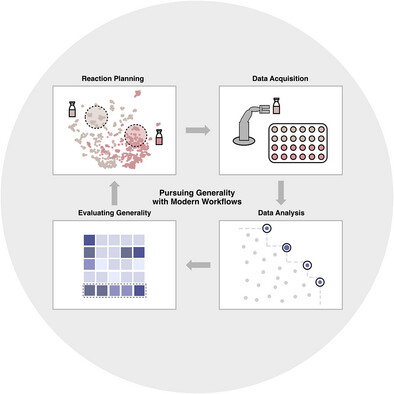
103. “Considerations in Pursuing Reaction Scope Generality” Zacate, S. B.; Dantas, J. A.; Lin, S.; Doyle, A. G.; Sigman, M. S. Angew. Chem. Int. Ed., 2025, 64, e202511091. [DOI:10.1002/anie.202511091] Link PDF
“Reactivity Studies of Bipyridine-Ligated Nickel(I) and Nickel(0) Complexes Inform the Mechanism in Modern Cross-Coupling Reactions” Raab, T. Judah.; Doyle, A. G. J. Am. Chem. Soc., 2025, 147, 33991–34000. [DOI: 10.1021/jacs.5c11247] Link PDF
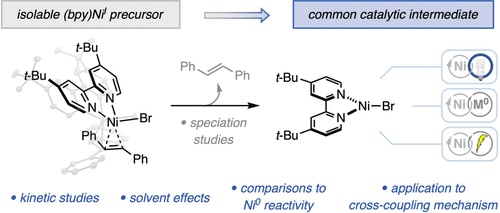
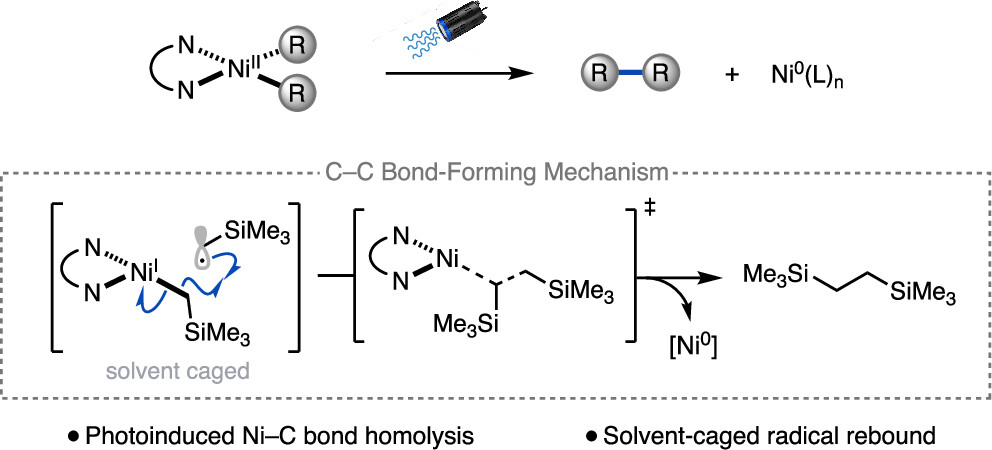
“Light-Promoted C(sp³)–C(sp³) Reductive Elimination from Dialkyl Ni(II) Complexes” Cusumano, A. Q.; Chaffin, B. C.; Cagan, D. A.; DiLuzio, S.; Sutcliffe, E.; Hadt, R. G.; Doyle, A. G. J. Am. Chem. Soc., 2025, 147, 32941–32950. [DOI: 10.1021/jacs.5c09925] Link PDF
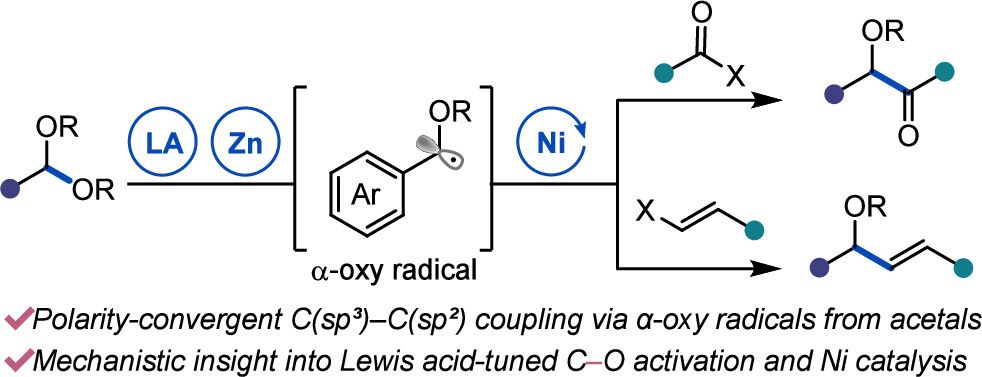
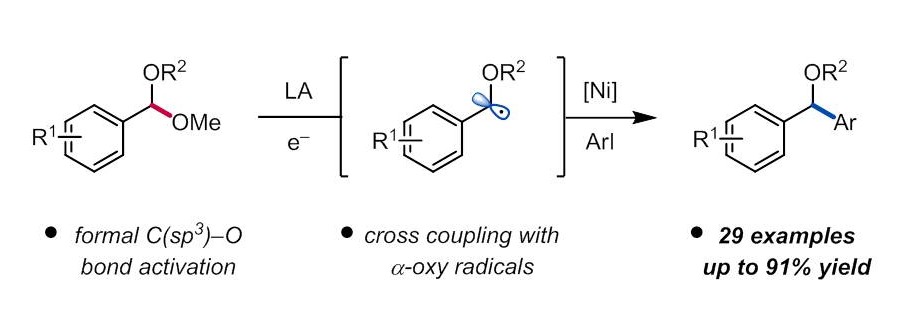
“Ni-Catalyzed Reductive Coupling of Acetals with Anhydrides and Vinyl Triflates via Single-Electron C–O Activation” Kim, E.; Borden, M. A.; Hwang, J.; Doyle, A. G.; Dongbang, S. Org. Lett., 2025, 27, 9454–9459. [DOI: 10.1021/acs.orglett.5c02788] Link PDF
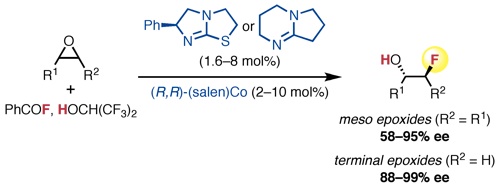
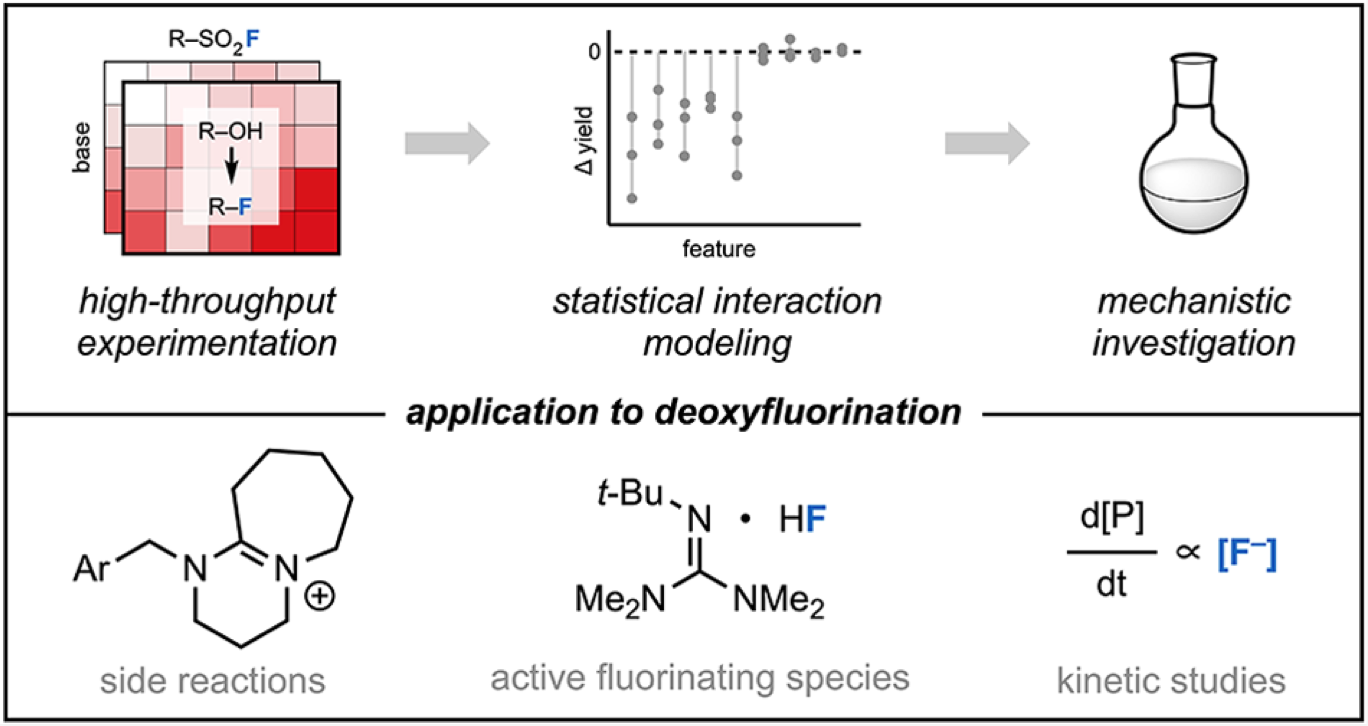
“Data Science-Guided Development of Deoxyfluorination Reagents with Enhanced Reactivity, Practicality, and Safety” Ruos, M. R.; Romer, N. P.; Deichert, J. A.; Alabanza, L. M.; Gandhi, S. S.; Brown, G. Z.; Walroth, R. C.; Cruz, K.; Gosselin, F.; Hong, A. Y.; Sigman, M. S.; Doyle, A. G. J. Am. Chem. Soc., 2025, 147, 25815–25824. [DOI: 10.1021/jacs.5c07548] Link PDF

“Fabrication of SERS Substrates Using Silver-Coated Gold Nanostars for Chemical Sensing: A Multiobjective Bayesian Optimization Approach” Giordano, A. N.; Franqui-Rios, S.; Quarin, S. M.; Vang, D.; Austin, D. R.; Doyle, A. G.; Baldwin, L. A.; Strobbia, P.; Rao, R. ACS Appl. Nano Mater., 2025, 8, 11930-11939. [DOI: 10.1021/acsanm.5c01462] Link PDF

“Applying Active Learning toward Building a Generalizable Model for Ni-Photoredox Cross-Electrophile Coupling of Aryl and Alkyl Bromides” Souza, L. W.; Ricke, N. D.; Chaffin, B. C.; Fortunato, M. E.; Jiang, S.; Soylu, C.; Caya, T. C.; Lau, S. H.; Wieser, K. A.; Doyle, A. G.; Tan, K. L. J. Am. Chem. Soc., 2025, 147, 18747-18759. [DOI: 10.1021/jacs.5c02218] Link PDF

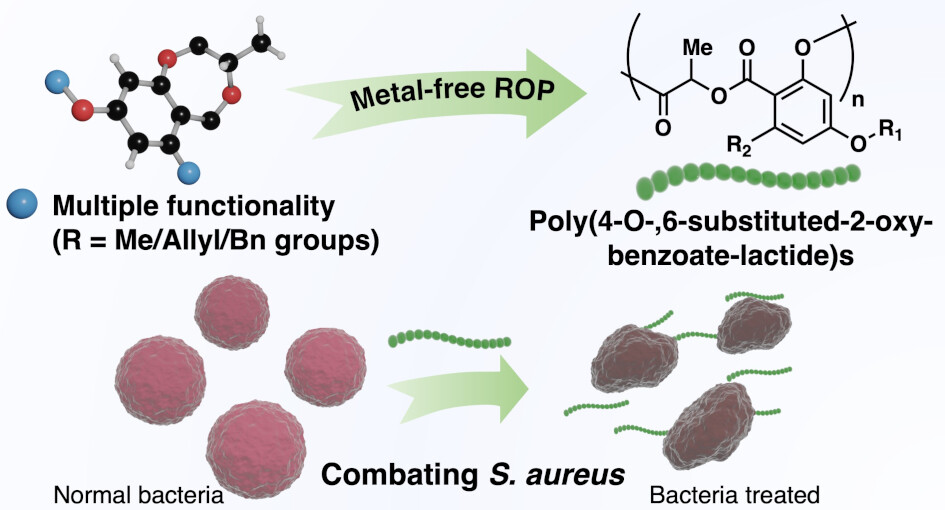
“Biosourced Functional Hydroxybenzoate-co-Lactide Polymers with Antimicrobial Activity” Salas-Ambrosio, P.; Vexler, S.; Sivasankaran, R. P.; Vlahakis, N.; Lai, R. S.; Johnson, C.; Baas-Maynard, S. I.; Min, D. S.; Lower, H.; Doyle, A. G.; Tang, Y.; Rodriguez, J. A.; Chen, I. A.; Read de Alaniz, J.; Maynard, H. D. J. Am. Chem. Soc., 2025, 147, 19230-19238. [DOI: 10.1021/jacs.5c04624] Link PDF
“Diversification of acridinium photocatalysts: Property tuning and reactivity in model reactions” Wang, J. Y.; Fan, F.; Ruos, M. E.; Adao Gomes, L.; Lavin, M.; O’Connor, T. J.; Lopez, S. A.; Doyle, A. G. Tet. Lett., 2025, 160, 155546. [DOI: 10.1016/j.tetlet.2025.155546] Link PDF
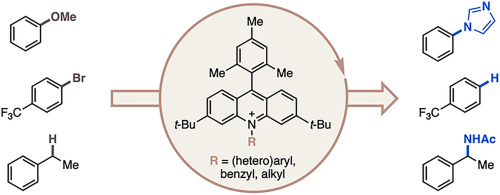
“Data Science-Driven Discovery of Optimal Conditions and a Condition-Selection Model for the Chan–Lam Coupling of Primary Sulfonamides” Gandhi, S. S.; Brown, G. Z.; Aikonen, S.; Compton, J. S.; Neves, P.; Martinez Alvarado, J. I.; Strambeanu, I. I.; Leonard, K. A.; Doyle, A. G. ACS Catal., 2025, 15, 2292-2304. [DOI: 10.1021/acscatal.4c07972] Link PDF
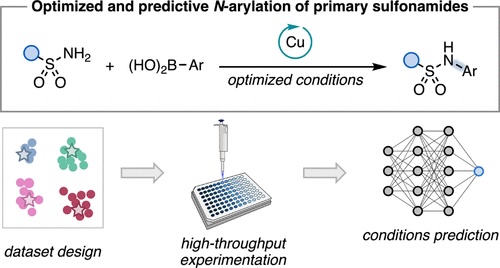
“Multi-Threshold Analysis for Chemical Space Mapping of Ni-Catalyzed Suzuki-Miyaura Couplings” LeSueur, A.; Tao, N.; Doyle, A. G.; Sigman, M. Eur. J. Org. Chem., 2024, e202400428. [DOI: 10.1002/ejoc.202400428] Link PDF
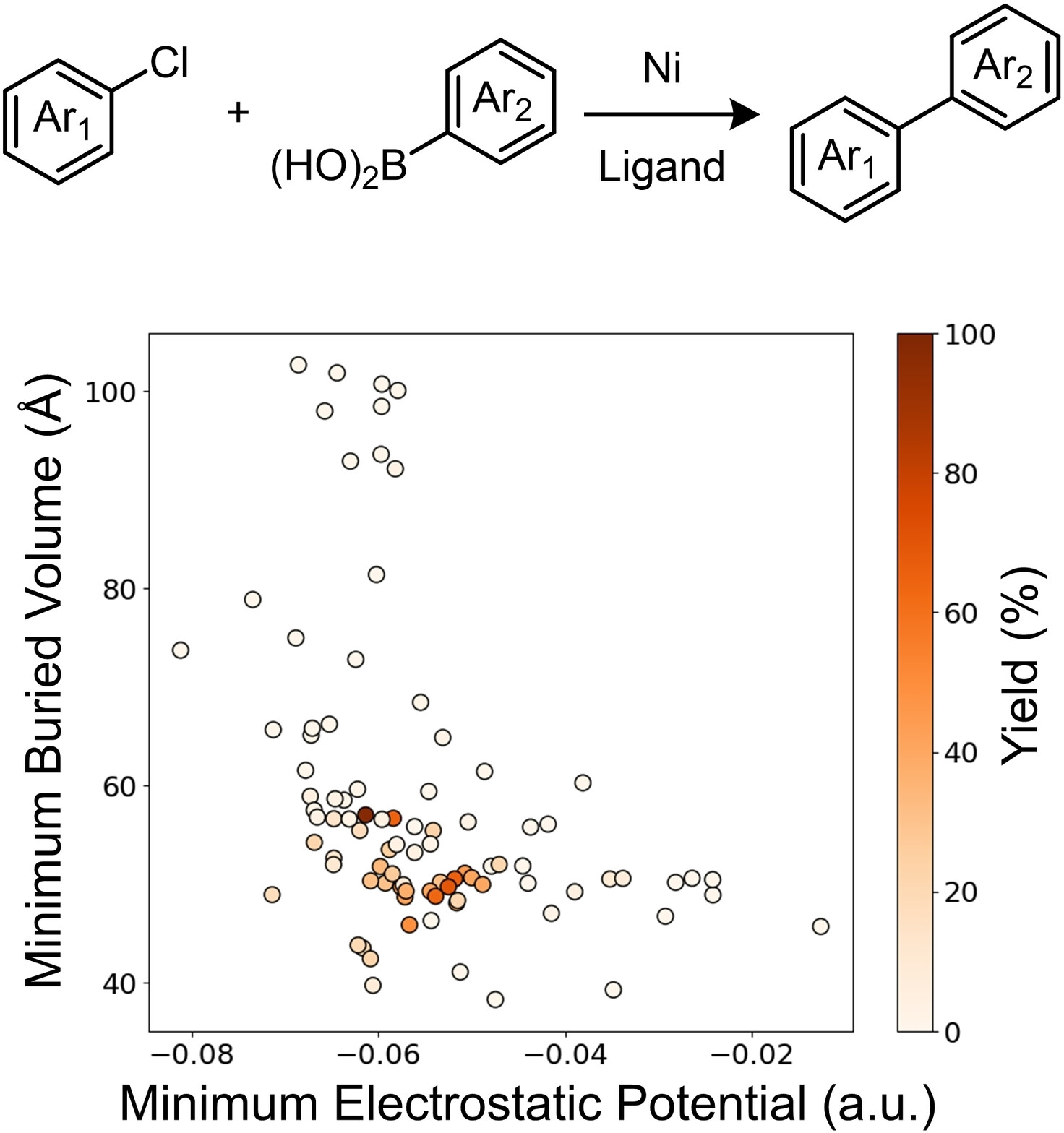
“Cooperative Phosphine-Photoredox Catalysis Enables N–H Activation of Azoles for Intermolecular Olefin Hydroamination” Sedillo, K.; Fan, F.; Knowles, R. R.; Doyle, A. G. J. Am. Chem. Soc., 2024, 146, 20349-20356. [DOI: 10.1021/jacs.4c05881] Link PDF
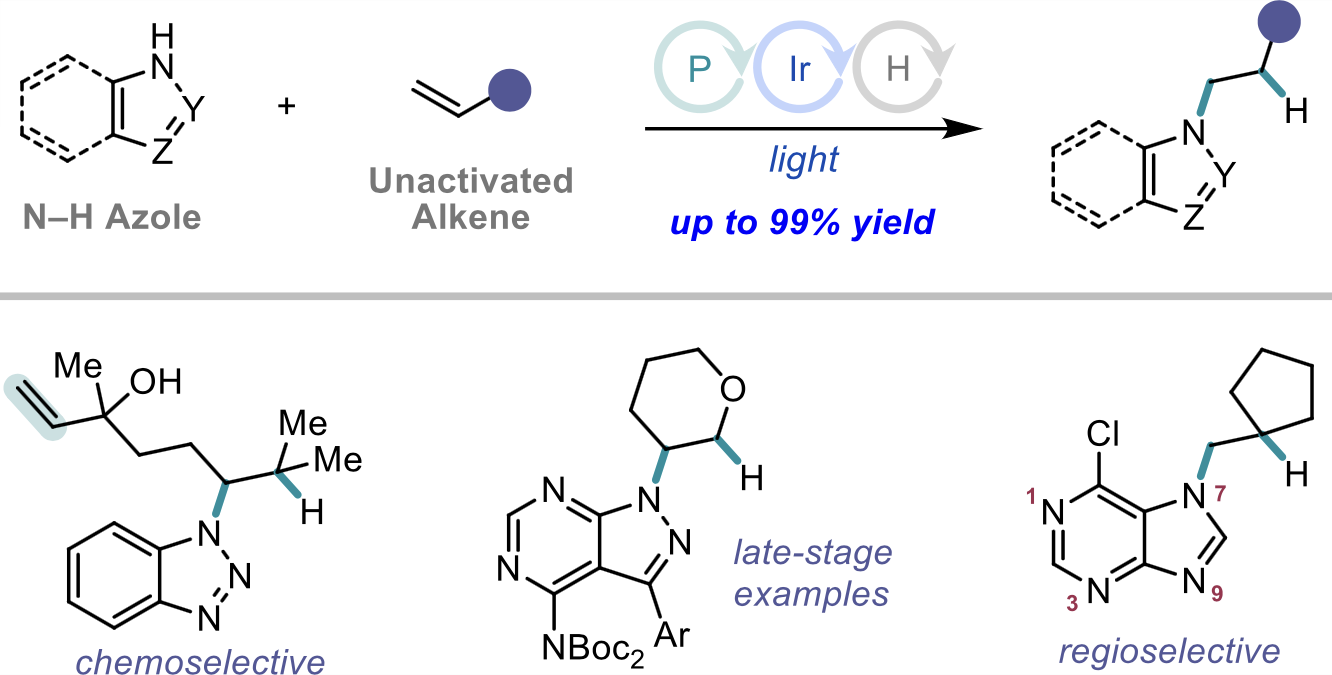
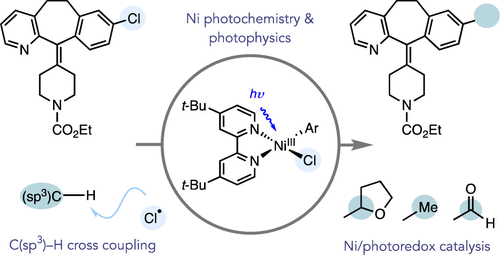
“Mechanism of Ni-Catalyzed Photochemical Halogen Atom-Mediated C(sp3)–H Arylation” Cusumano, A. Q; Chaffin, B. C.; Doyle, A. G. J. Am. Chem. Soc., 2024, 146, 15331-15344. [DOI: 10.1021/jacs.4c03099] Link PDF
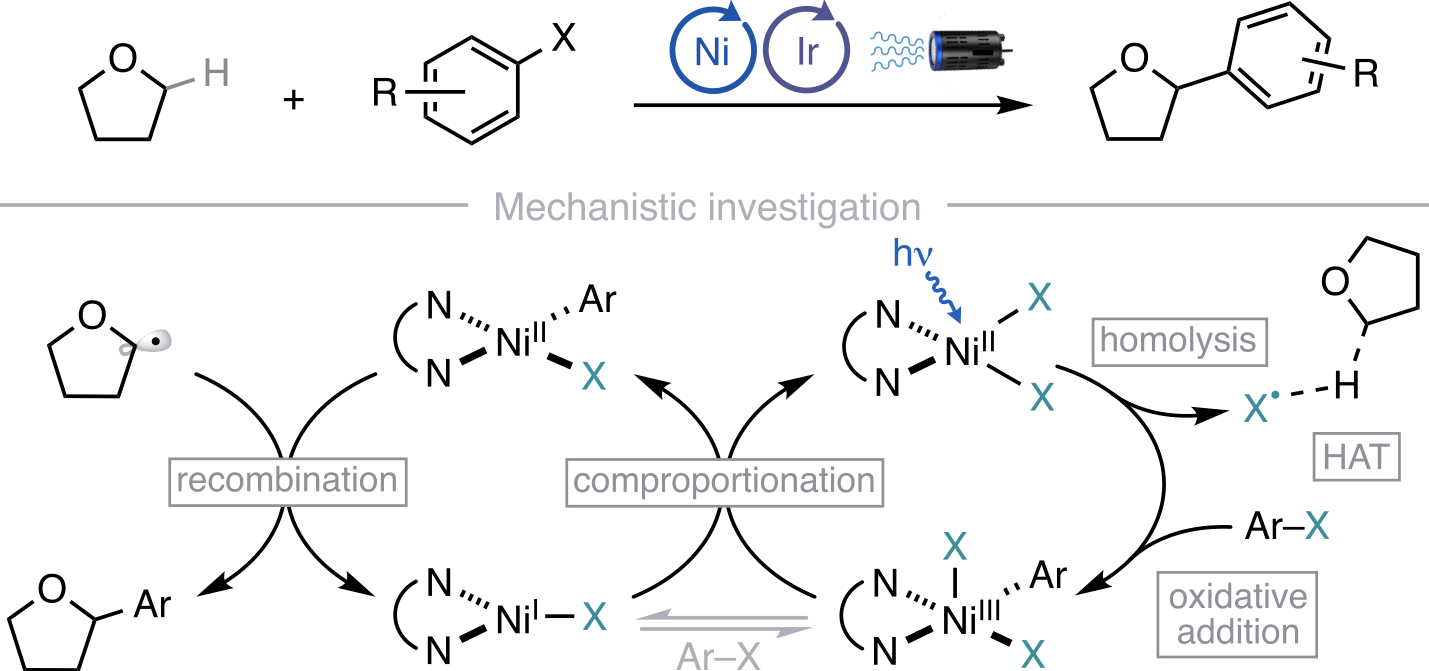
“Autonomous closed-loop mechanistic investigation of molecular electrochemistry via automation” Sheng, H.; Sun, J.; Rodríguez, O.; Hoar, B. B.; Zhang, W.; Xiang, D.; Tang, T.; Hazra, A.; Min, D. S.; Doyle, A. G.; Sigman, M. S.; Costentin, C.; Gu, Q.; Rodríguez-López, J.; Liu, C. Nat. Commun, 2024, 15, 2781. [DOI: 10.1038/s41467-024-47210-x] Link PDF
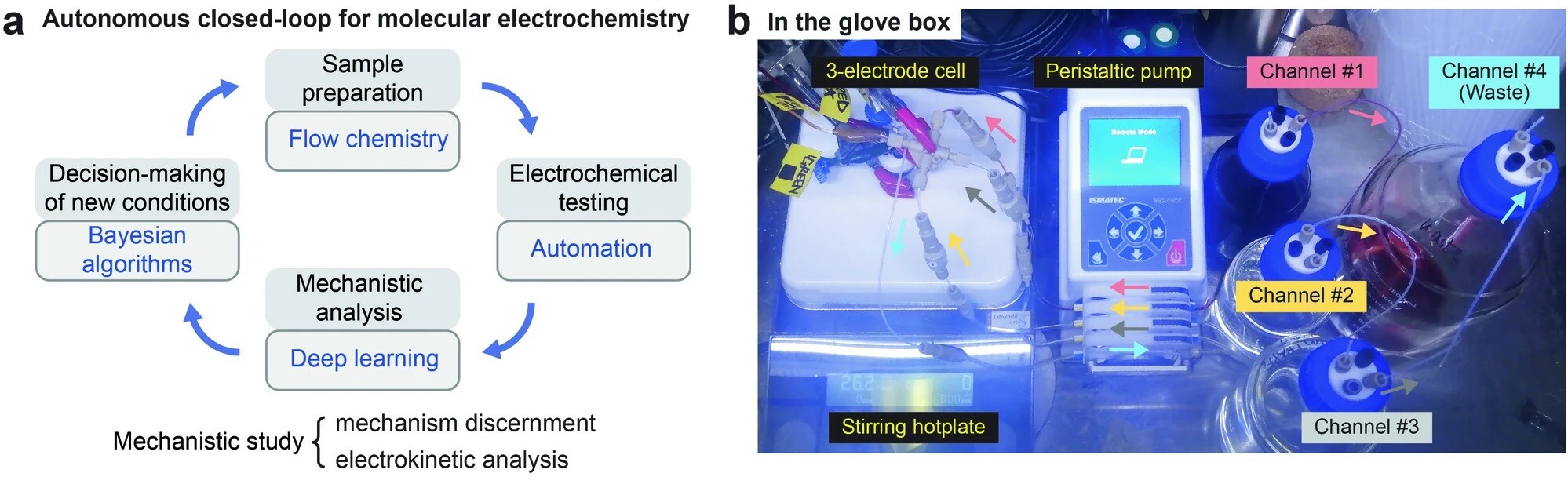
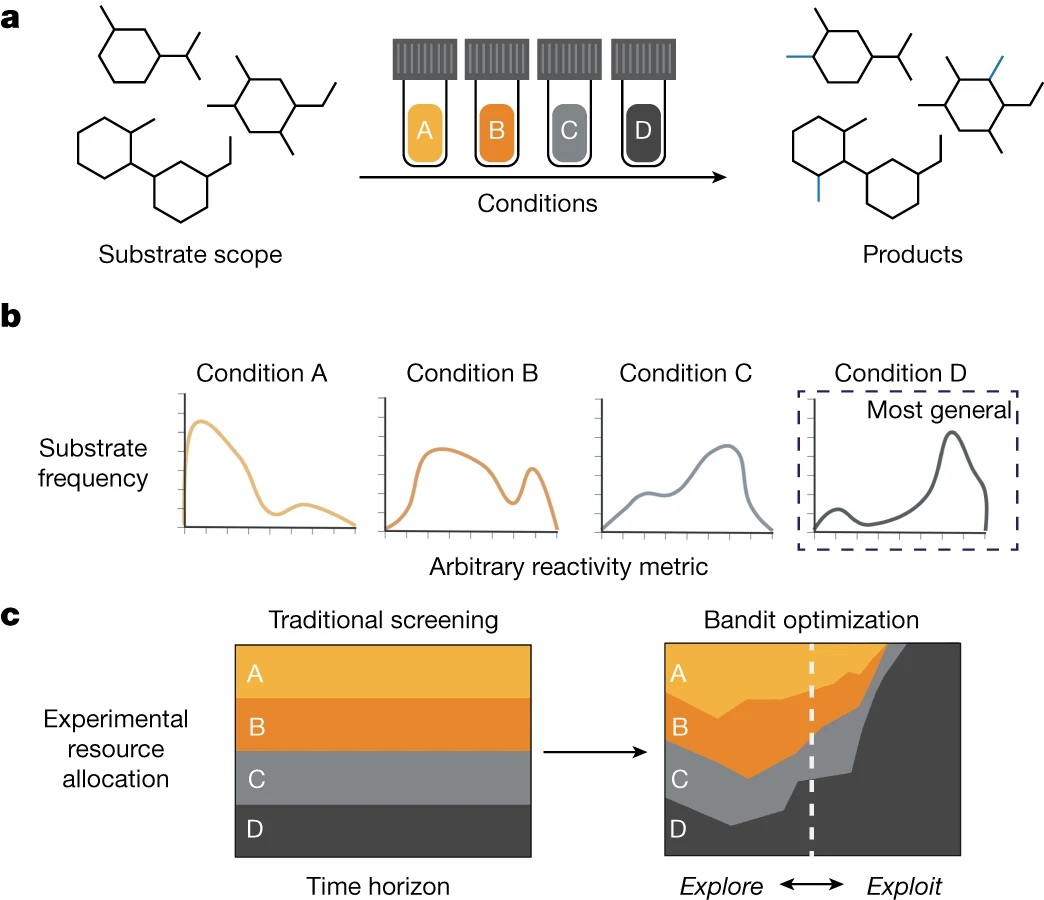
“Data Science Guided Multiobjective Optimization of a Stereoconvergent Nickel-Catalyzed Reduction of Enol Tosylates to Access Trisubstituted Alkenes” Romer, N. P.; Min, D. S.; Wang, J. Y.; Walroth, R. C.; Mack, K. A.; Sirois, L. E.; Gosselin, F.; Zell, D.; Doyle, A. G.; Sigman, M. S. ACS Catal, 2024, 14, 4699-4708. [DOI: 10.1021/acscatal.4c00650] Link PDF
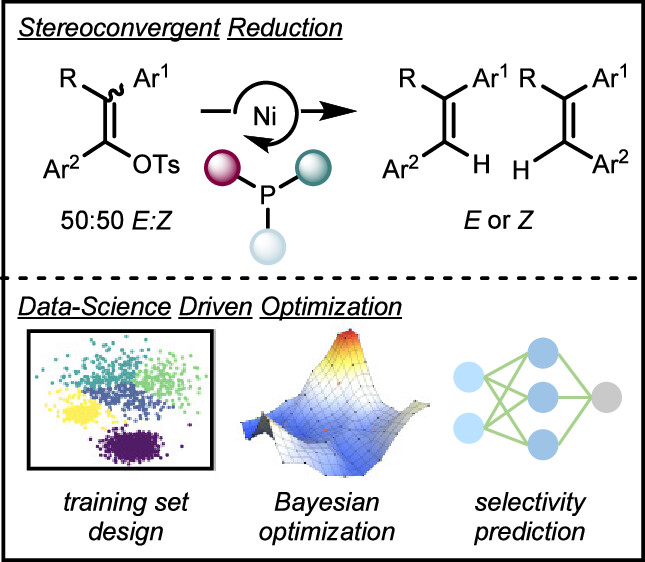
“Identifying general reaction conditions by bandit optimization” Wang, J. Y.; Stevens, J. M.; Kariofillis, S. K.; Tom, M.-J.; Golden, D. L.; Li, J.; Tabora, J. E.; Parasram, M.; Shields, B. J.; Primer, D. N.; Hao, B.; Del Valle, D.; DiSomma, S.; Furman, A.; Zipp, G. G.; Melnikov, S.; Paulson, J.; Doyle, A. G. Nature, 2024, 626, 1025-1033. [DOI: 10.1038/s41586-024-07021-y] Link PDF
“Broad Survey of Selectivity in the Heterogeneous Hydrogenation of Heterocycles” Lyons, T. W.; Leibler, I. N.-M.; He, C. Q.; Gadamsetty, S.; Estrada, G. J.; Doyle, A. G. J. Org. Chem. 2024, 89, 1438-1445.
[DOI: 10.1021/acs.joc.3c02028] Link PDF
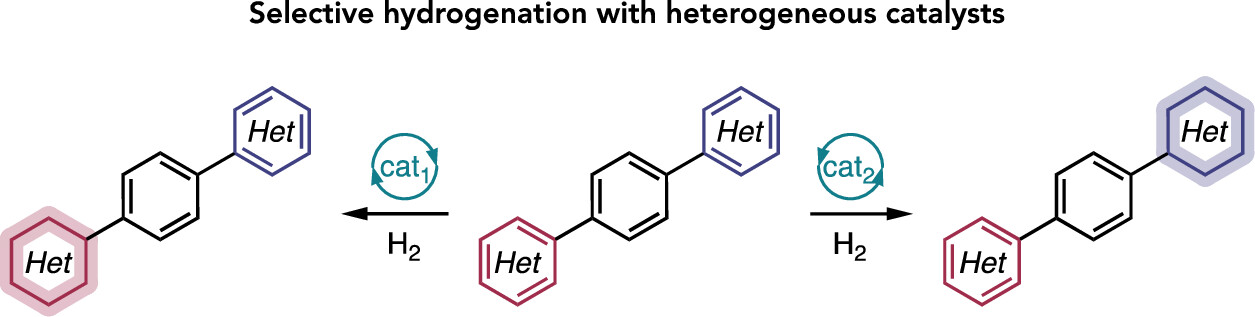
“Catalyst Deactivation of a Monoligated CyJohnPhos-Bound Nickel(0) Complex” Newman-Stonebraker, S. H.; Raab, T. J.; Doyle, A. G. Organometallics 2023, 42, 3438-3441.
[DOI: 10.1021/jacs.3c08301] Link PDF
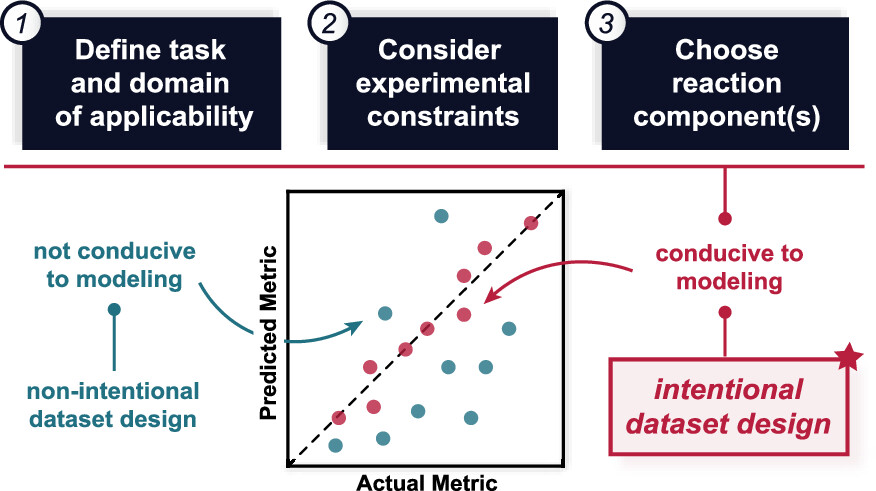
“Dataset Design for Building Models of Chemical Reactivity” Raghavan, P; Haas, B. C.; Ruos, M. E.; Schleinitz, J; Doyle, A. G.; Reisman, S. E.; Sigman, M. S.; Coley, C. W. J. Am. Chem. Soc. 2023, 145, 24175-24183.
[DOI: 10.1021/jacs.3c08301] Link PDF
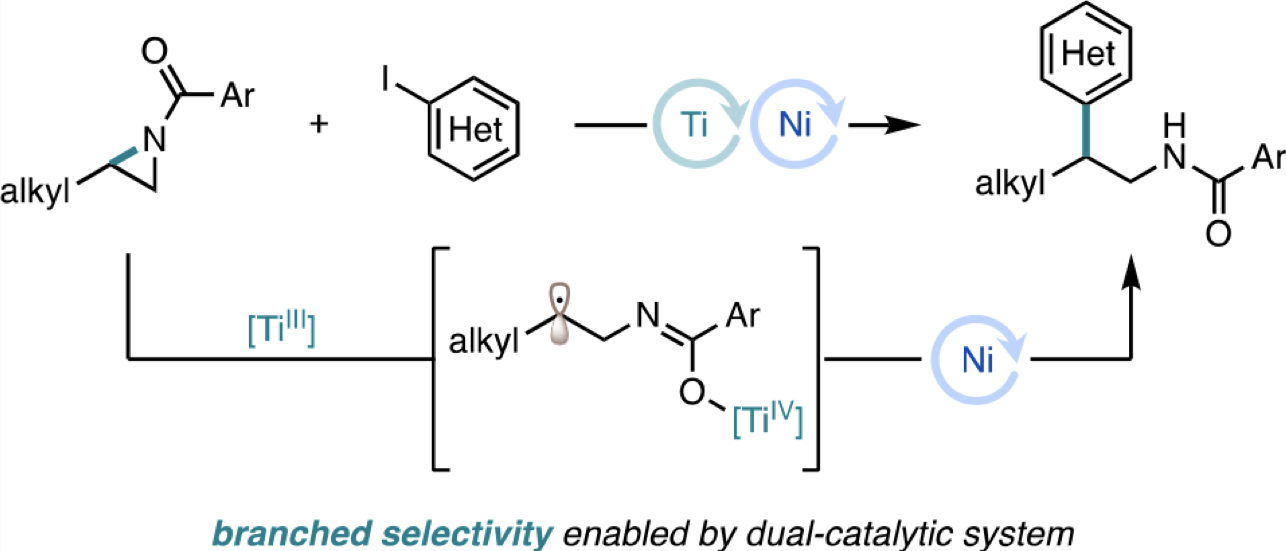
“Branched-Selective Cross-Electrophile Coupling of 2-Alkyl Aziridines and (Hetero)aryl Iodides Using Ti/Ni Catalysis” Williams, W. L.; Gutiérrez-Valencia, N. E.; Doyle, A. G. J. Am. Chem. Soc. 2023, 145, 24175-24183.
[DOI: 10.1021/jacs.3c08301] Link PDF
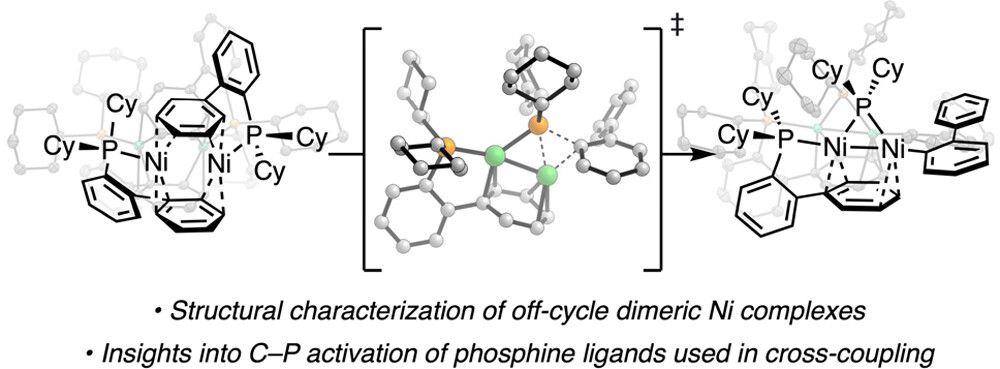
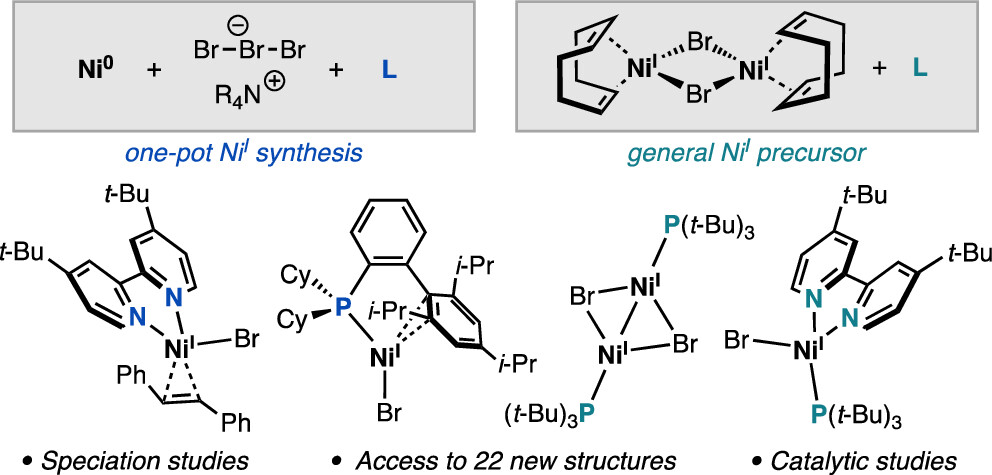
“Synthesis of Nickel(I)–Bromide Complexes via Oxidation and Ligand Displacement: Evaluation of Ligand Effects on Speciation and Reactivity” Newman-Stonebraker, S. H.; Raab, T. J.; Roshandel, H. R.; Doyle, A. G. J. Am. Chem. Soc. 2023, 145, 19368-19377.
[DOI: 10.1021/jacs.3c06233] Link PDF
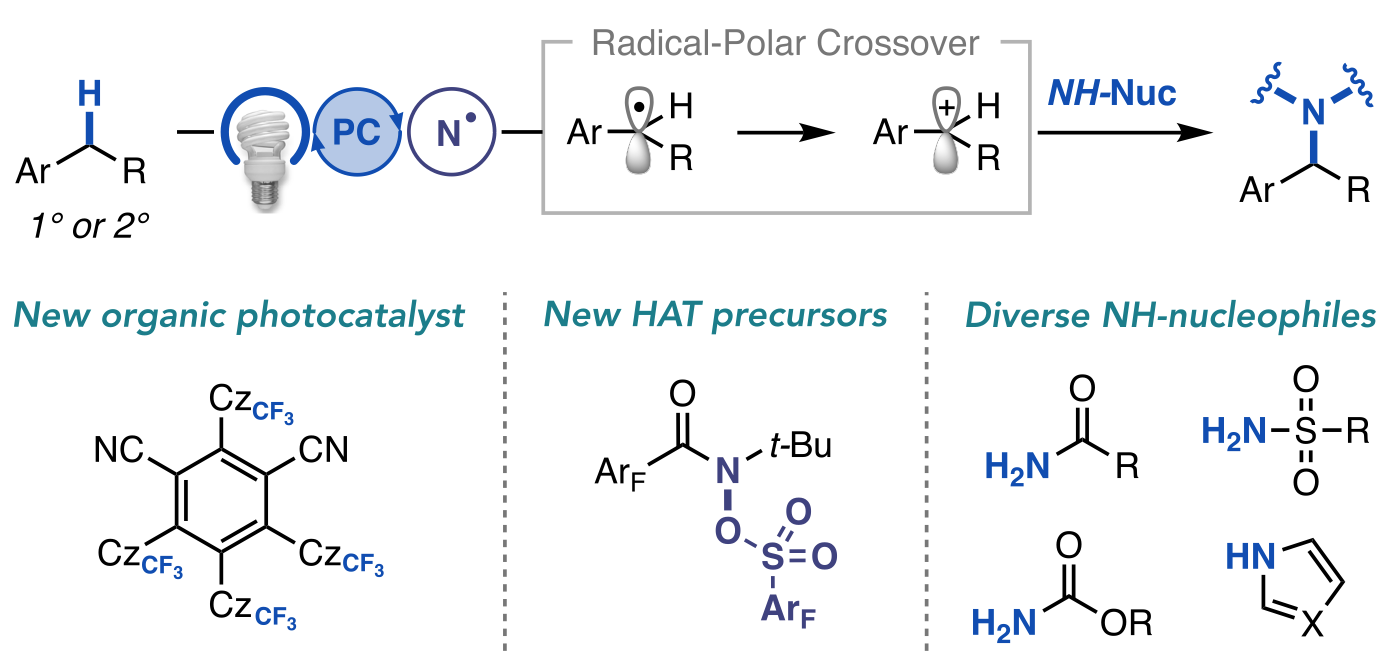
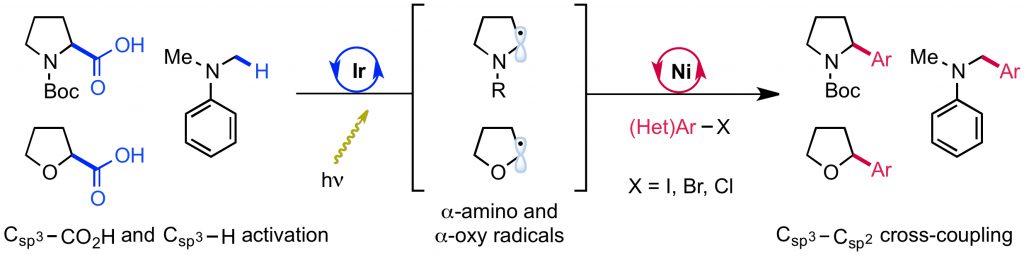
“A General Photocatalytic Strategy for Nucleophilic Amination of Primary and Secondary Benzylic C–H Bonds” Ruos, M. E.; Kinney, R. G.; Ring, O. T.; Doyle, A. G. J. Am. Chem. Soc. 2023, 145, 18487-18496.
[DOI: 10.1021/jacs.3c04912] Link PDF
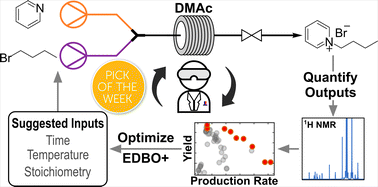
“Continuous flow synthesis of pyridinium salts accelerated by multi-objective Bayesian optimization with active learning” Dunlap, J. H.; Ethier, J. G.; Putnam-Neeb, A. A.; Iyer, S.; Luo, S.-X. L.; Feng, H.; Torres, J. A. G.; Doyle, A. G.; Swager, T. M.; Vaia, R. A.; Mirau, P.; Crouse, C. A.; Baldwin, L. A. Chem. Sci. 2023, 14, 8061-8069.
[DOI: 10.1039/d3sc01303k] Link PDF
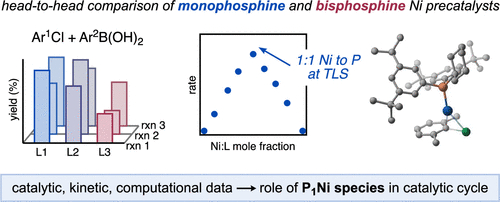
Borowski, J. E.; Newman-Stonebraker, S. H.; Doyle, A. G. ACS. Catal. 2023, 13, 7966-7977.
[DOI: 10.1021/acscatal.3c01331] Link PDF
Leibler, I. N.-M.; Gandhi, S. S.; Tekle-Smith, M. A.; Doyle, A. G. J. Am. Chem. Soc. 2023, 145, 9928-9950.
[DOI: 10.1021/jacs.3c01824] Link PDF
Tang, T.; Hazra, A.; Min, D. S.; Williams, W. L.; Doyle, A. G.; Sigman M. S. J. Am. Chem. Soc. 2023, 145, 8689-8699.
[DOI: 10.1021/jacs.3c01726] Link PDF
Żurański, A. M.; Gandhi, S. S.; Doyle, A. G. J. Am. Chem. Soc. 2023, 145, 7898–7909.
[DOI: 10.1021/jacs.2c13093] Link PDF
Saebi, M.; Nan, B.; Herr, J. E.; Wahlers, J.; Guo, Z.; Zurański, A. M.; Kogej, T.; Norrby, P.-O.; Doyle, A. G.; Wiest, O.; Chawla, N. V. Chem. Sci. 2023.
[DOI: 10.1039/d2sc06041h] Link PDF
Murray, P. R. D.; Leibler, I. N.-M.; Hell, S. M.; Villalona, E.; Doyle, A. G.; Knowles, R. R. ACS Catal. 2022, 12, 13732-13740.
[DOI: 10.1021/acscatal.2c04316] Link PDF
Torres, J. A. G.; Lau, S. H.; Anchuri, P.; Stevens, J. M.; Tabora, J. E.; Li, J.; Borovika, A.; Adams, R. P.; Doyle, A. G. J. Am. Chem. Soc. 2022, 144, 19999-20007.
[DOI: 10.1021/jacs.2c08592] Link PDF Link EDBO+ web app.
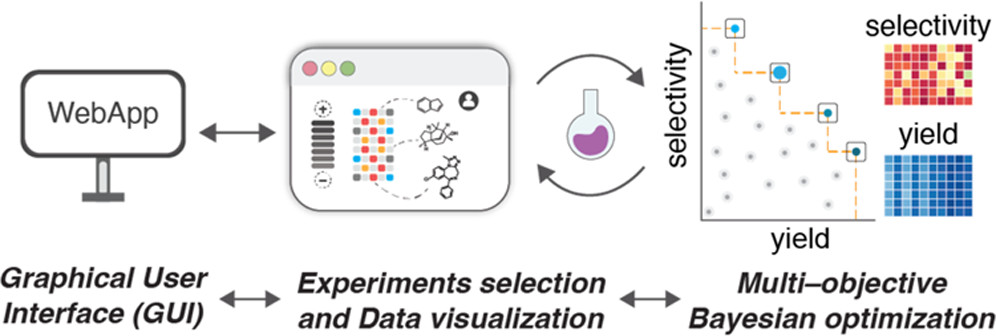
Dongbang, S; Doyle, A. G. J. Am. Chem. Soc. 2022, 144, 20067–20077.
[DOI: 10.1021/jacs.2c09294] Link PDF
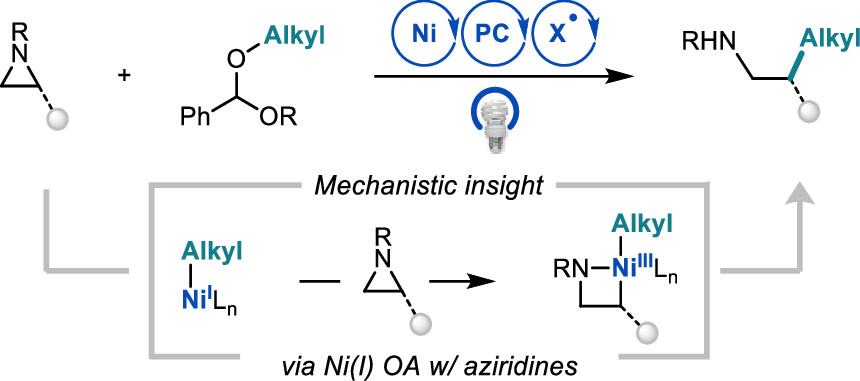
Newman-Stonebraker, S. H.; Wang, J. Y.; Jeffrey P. D.; Doyle, A. G. J. Am. Chem. Soc. 2022, 144, 19635-19648.
[DOI: 10.1021/jacs.2c09840] Link PDF
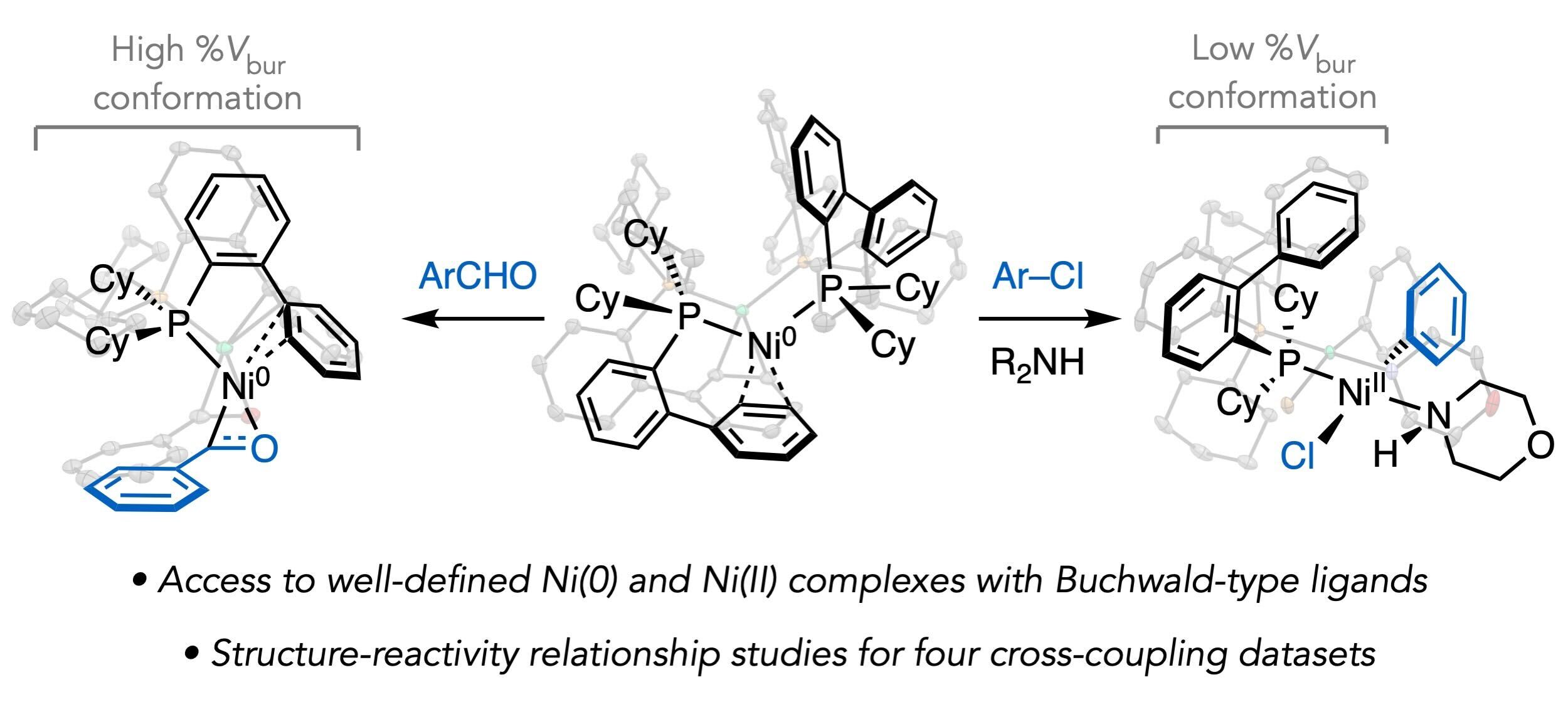
Millet, A.; Cesana, P. T.; Sedillo, K.; Bird, M. J.; Schlau-Cohen, G. S.; Doyle, A. G.; MacMillan, D. W. C.; Scholes, G. D. Acc. Chem. Res. 2022, 55, 1423-1434.
[DOI: 10.1021/acs.accounts.2c00083] Link PDF
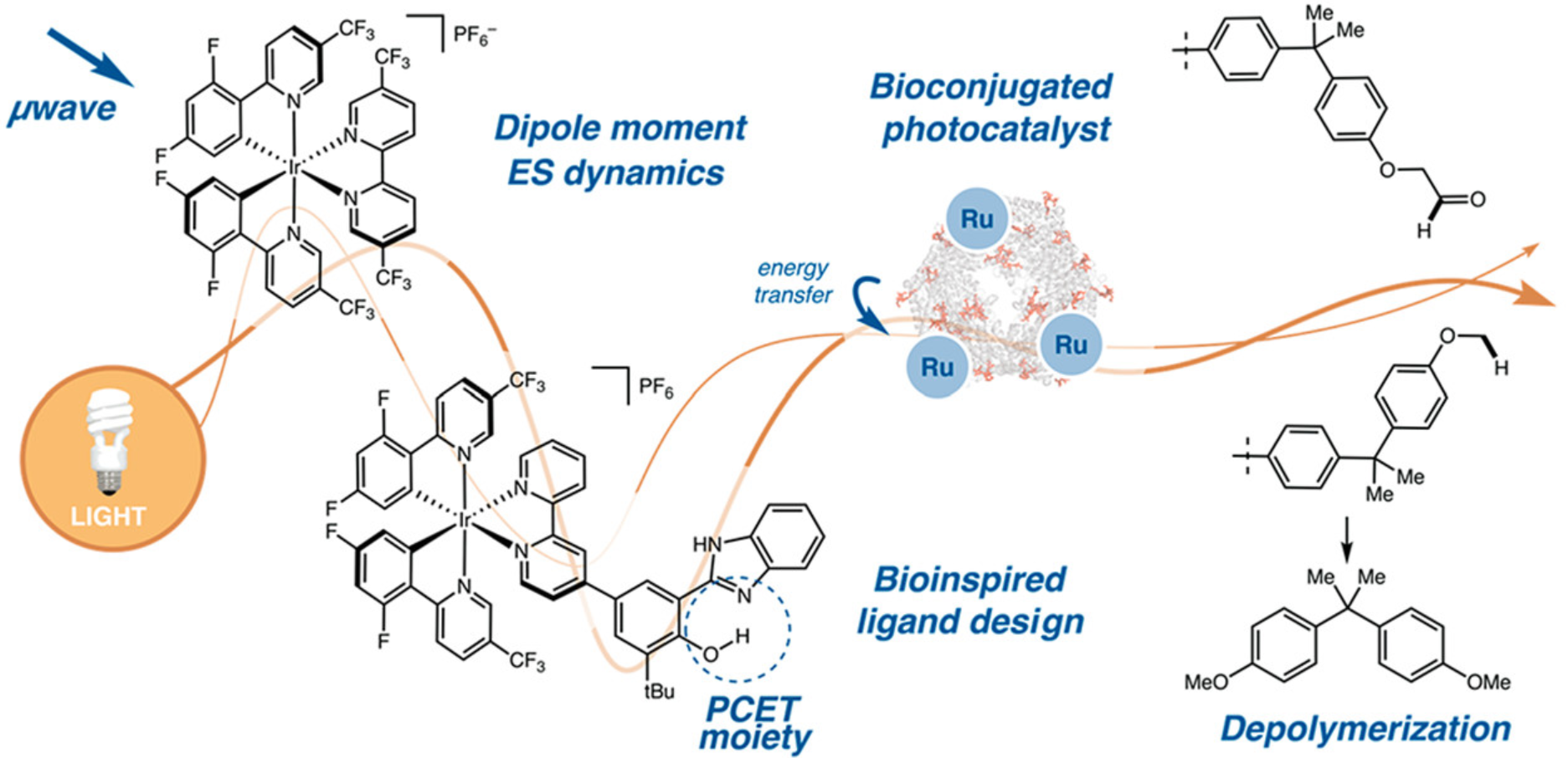
Ting, S. I.; Williams, W. L.; Doyle, A. G. J. Am. Chem. Soc. 2022, 144, 5575-5582.
[DOI: 10.1021/jacs.2c00462] Link PDF
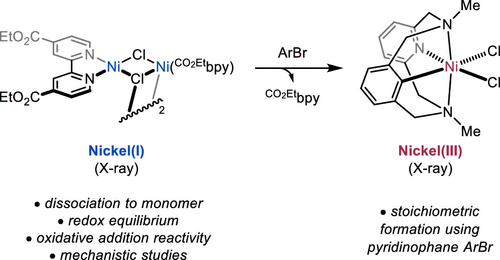
Żurański, A. M.; Wang, J. Y.; Shields, B. J.; Doyle, A. G. React. Chem. Eng. 2022, 7, 1276-1284.
[DOI: 10.1039/D2RE00030J] Link PDF
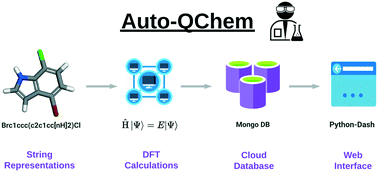
Kariofillis, S. K.; Jiang, S.; Żurański, A. M.; Gandhi, S. S.; Martinez Alvarado, J. I.; Doyle, A. G. J. Am. Chem. Soc. 2022, 144, 1045-1055.
[DOI: 10.1021/jacs.1c12203] Link PDF
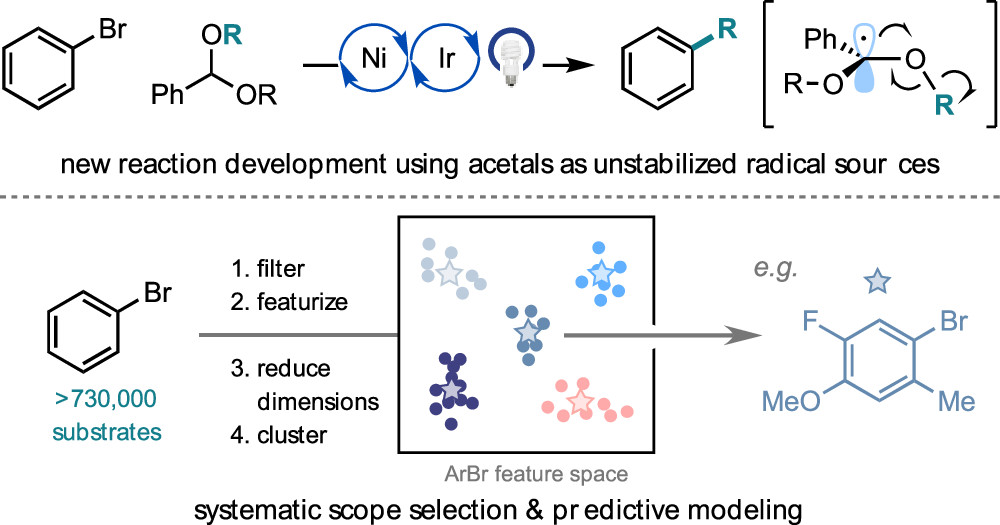
Leibler, I. N.-M.; Tekle-Smith, M. A.; Doyle, A. G. Nat. Commun. 2021, 12, 6950.
[DOI: 10.1038/s41467-021-27165-z] Link PDF
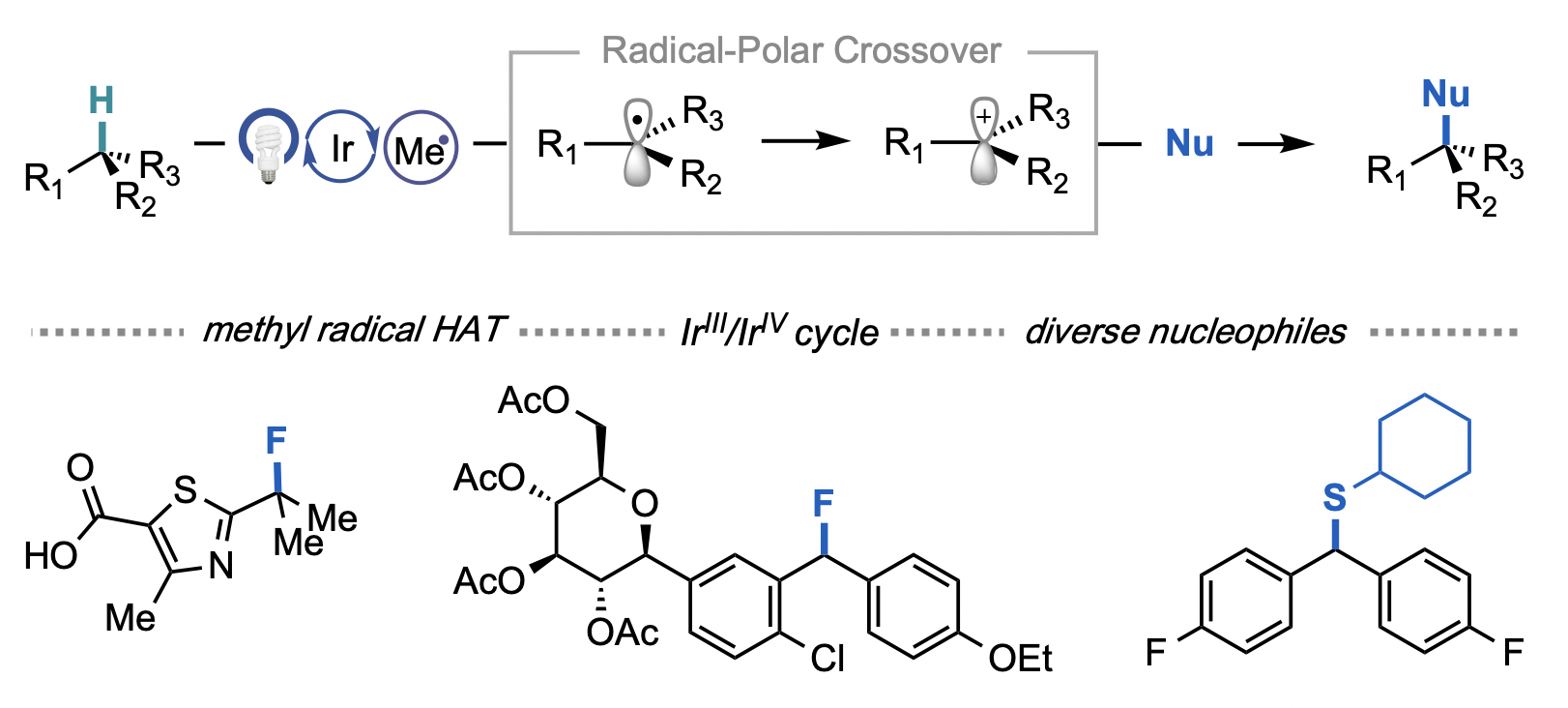
Cesana, P. T.; Li, B. X.; Shepard, S. G.; Ting, S. I.; Hart, S. M.; Olson, C. M.; Martinez Alvarado, J. I.; Son, M.; Steiman, T. J.; Castellano, F. N.; Doyle, A. G.; MacMillan, D. W. C.; Schlau-Cohen, G. S. Chem. 2021, 8, 174-185.
[DOI: 10.1016/j.chempr.2021.10.010] Link PDF
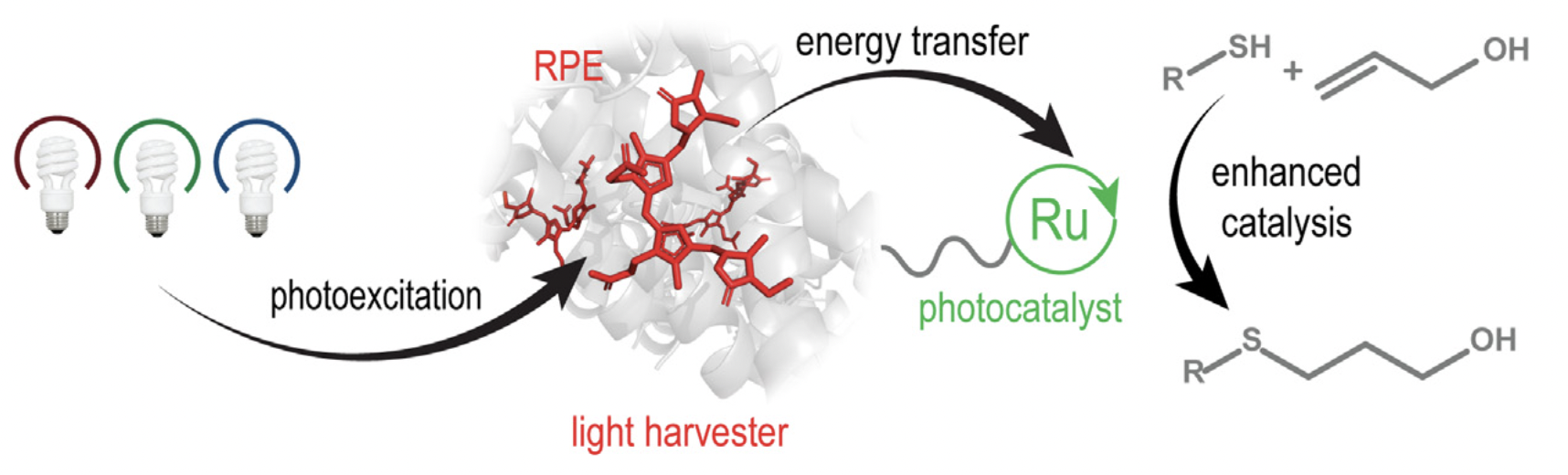
Kearnes, S. M.; Maser, M. R.; Wleklinski, M.; Kast, A.; Doyle, A. G.; Dreher, S. D.; Hawkins, J. M.; Jensen, K. F.; Coley, C. W. J. Am. Chem. Soc. 2021, 143, 18820-18826.
[DOI: 10.1021/jacs.1c09820] Link PDF
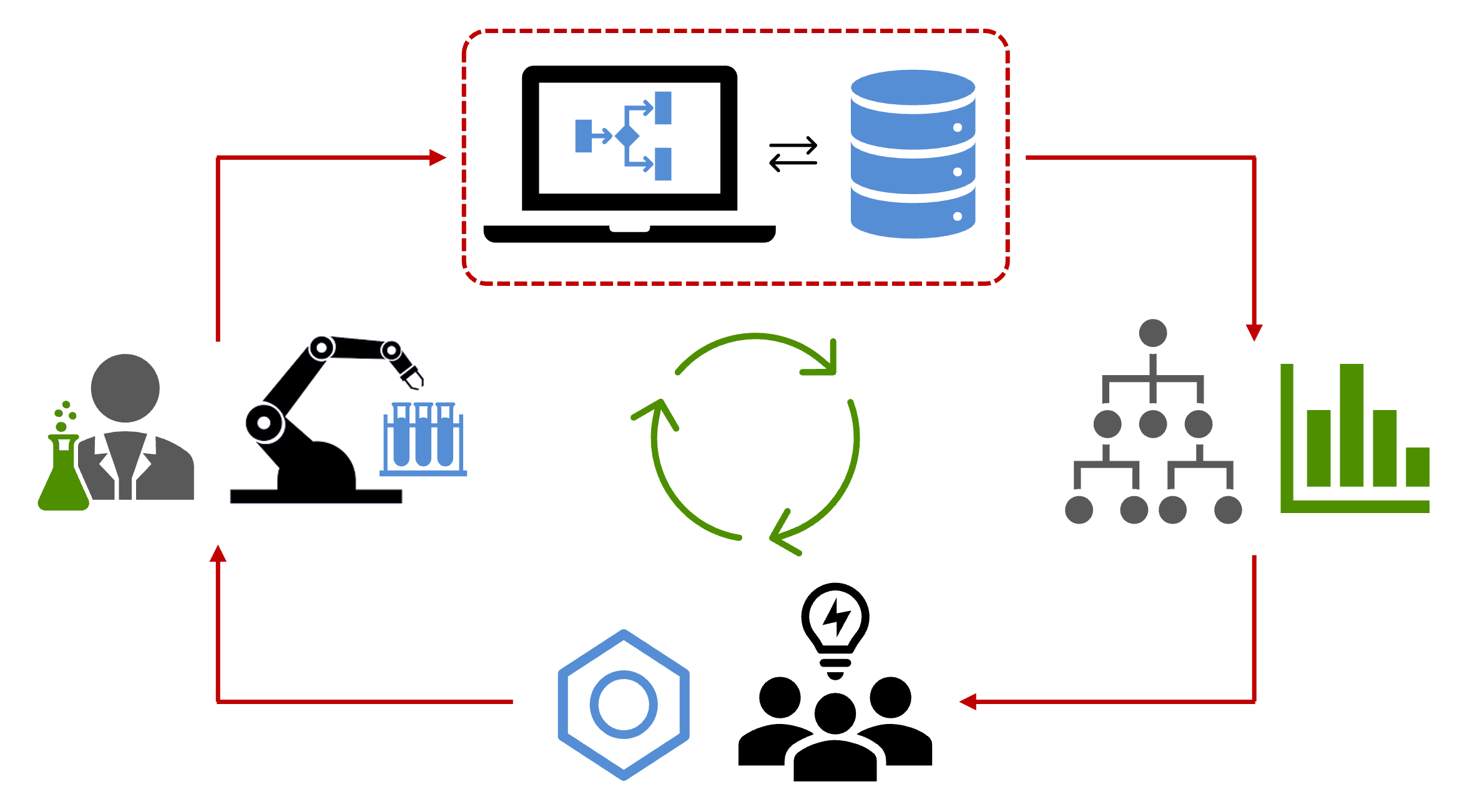
Williams, W. L.; Zeng, L.; Gensch, T.; Sigman, M. S.; Doyle, A. G.; Anslyn, E. V. ACS Cent. Sci. 2021, 7, 1622-1637.
[DOI: 10.1021/acscentsci.1c00535] Link PDF
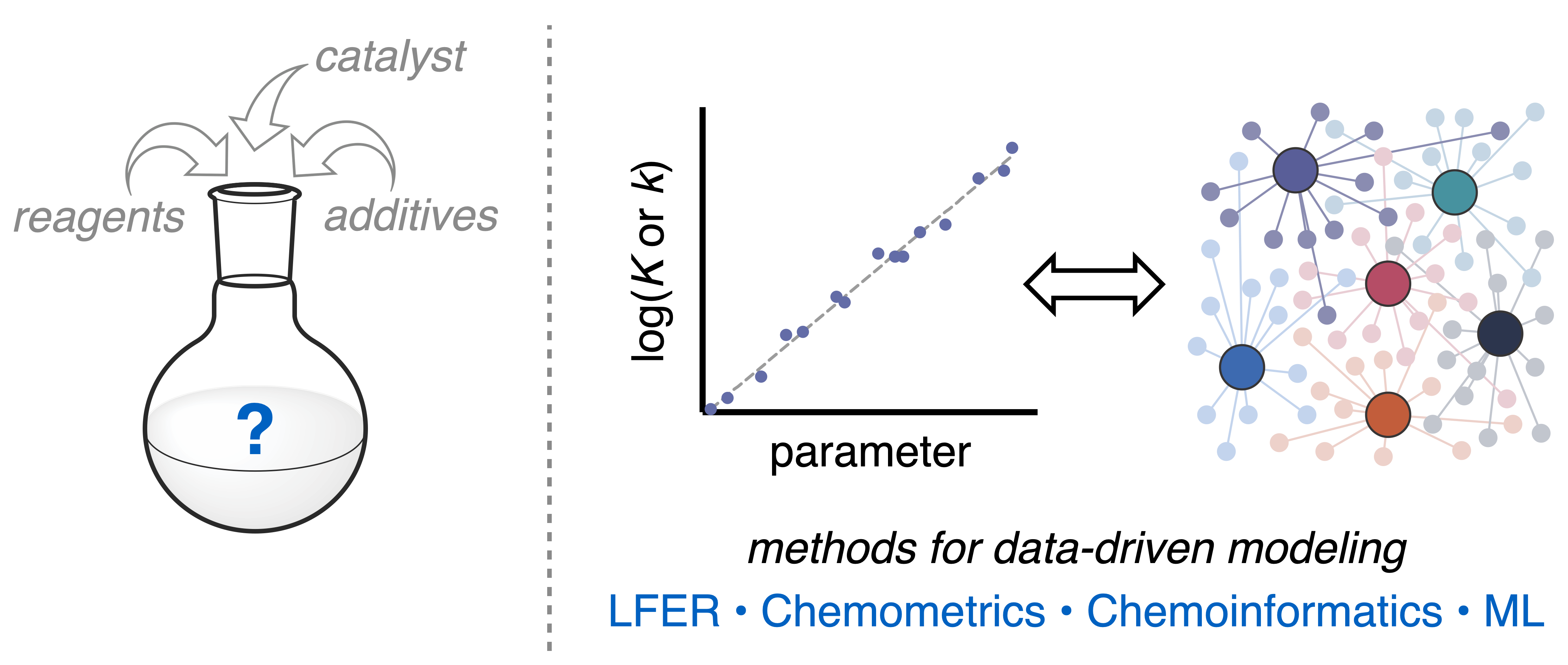
Chinn, A. J.; Sedillo, K.; Doyle, A. G. J. Am. Chem. Soc. 2021, 143, 18331-18338.
[DOI: 10.1021/jacs.1c09484] Link PDF
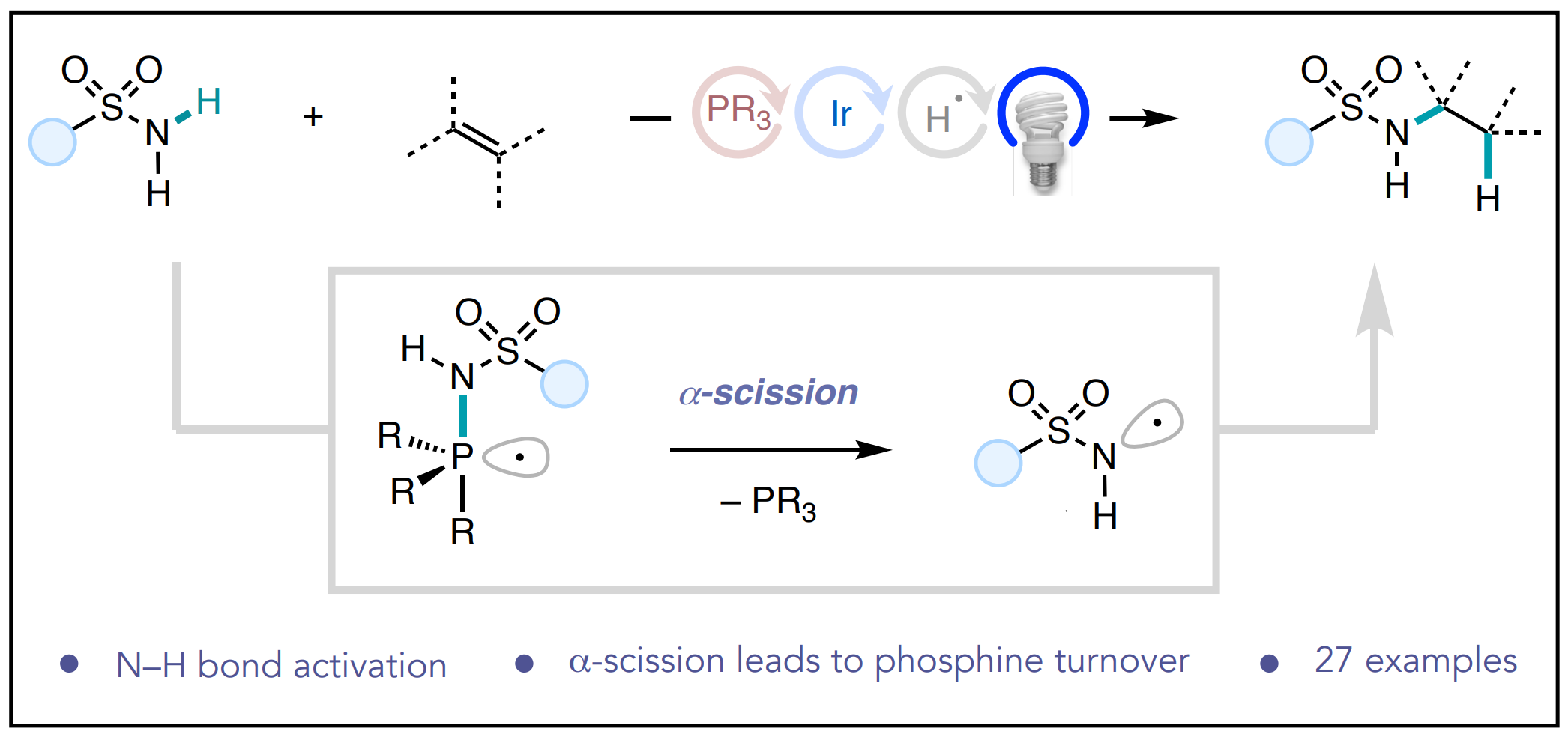
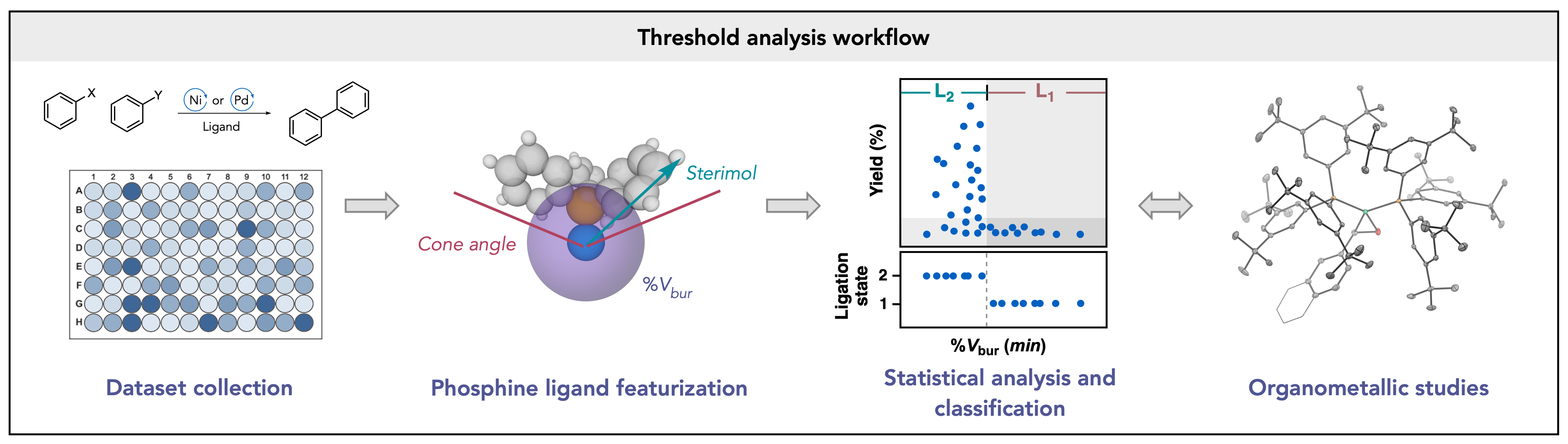
62. Univariate classification of phosphine ligation state and reactivity in cross-coupling catalysis
Newman-Stonebraker, S. H.; Smith, S. R.; Borowski, J. E.; Peters, E.; Gensch, T.; Johnson, H. C.; Sigman, M. S.; Doyle, A. G. Science 2021, 374, 301-308.
[DOI: 10.1126/science.ajb4213] Link PDF
Lau, S. H.; Borden, M. A.; Steiman, T. J.; Parasram, M.; Wang, L. S.; Doyle, A. G. J. Am. Chem. Soc. 2021, 143, 15873-15881.
[DOI: 10.1021/jacs.1c08105] Link PDF
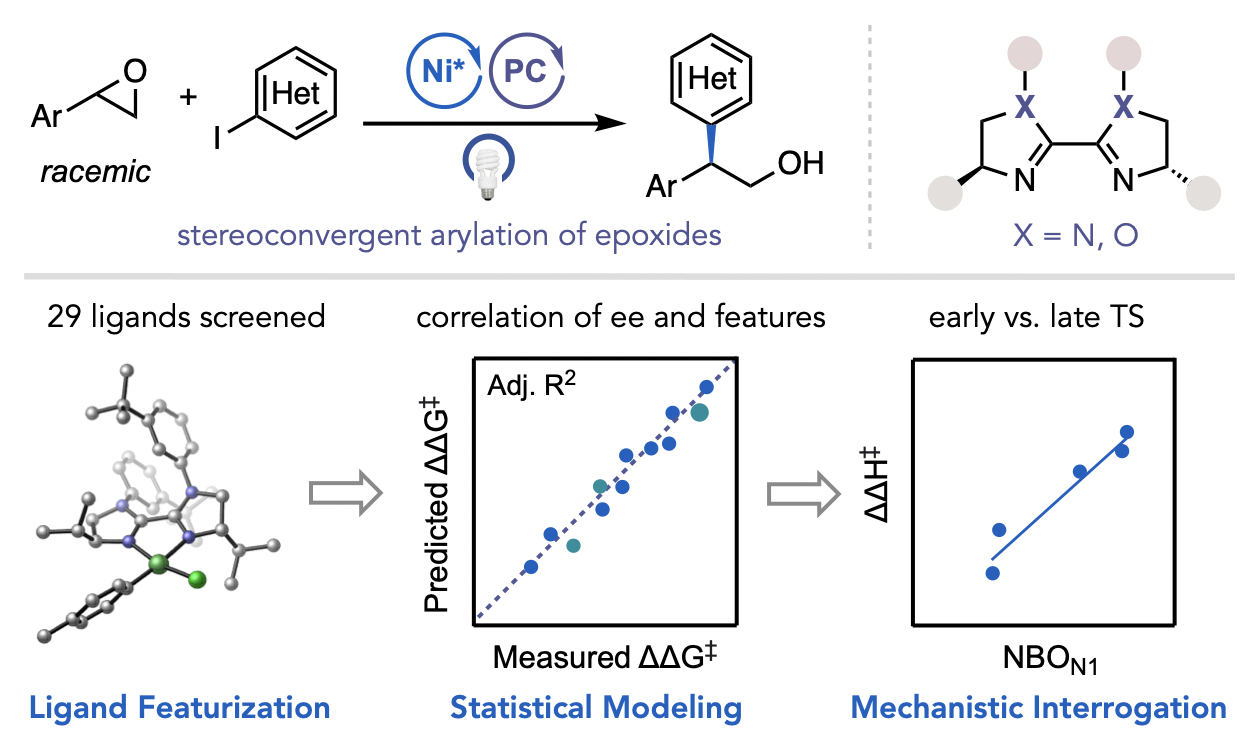
Żurański, A. M.; Martinez Alvarado, J. I.; Shields, B. J.; Doyle, A. G. Acc. Chem. Res. 2021, 54, 1856-1865.
[DOI: 10.1021/acs.accounts.0c00770] Link PDF
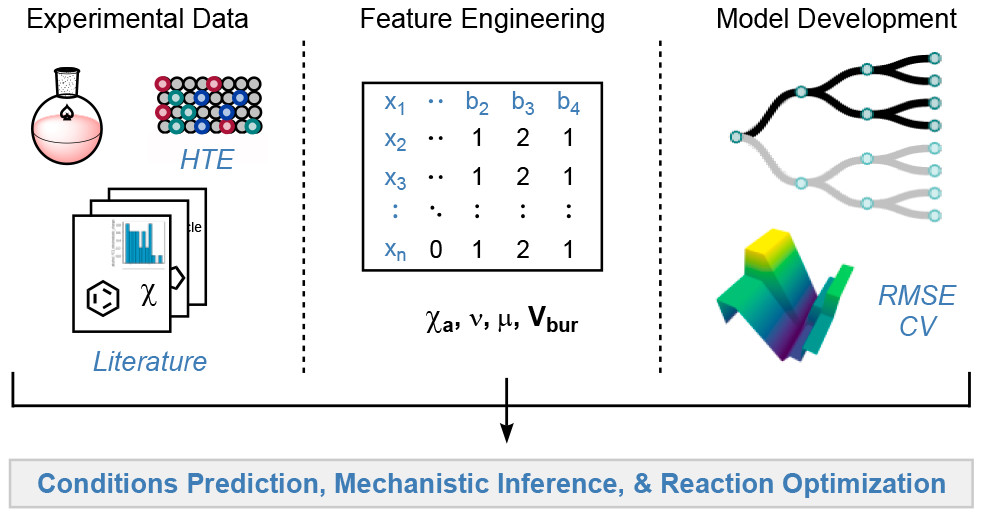
Shen, Y.; Borowski, J. E.; Hardy, M. A.; Sarpong, R.; Doyle, A. G.; Cernak, T. Nat. Rev. Methods Primers 2021, 1, 23.
[DOI: 10.1038/s43586-021-00022-5] Link PDF
Shields, B. J.; Stevens, J.; Li, J.; Parasram, M.; Damani, F.; Martinez Alvarado, J. I.; Janey, J. M.; Adams, R. P.; Doyle, A. G. Nature 2021, 590, 89-96.
[DOI: 10.1038/s41586-021-03213-y] Link PDF
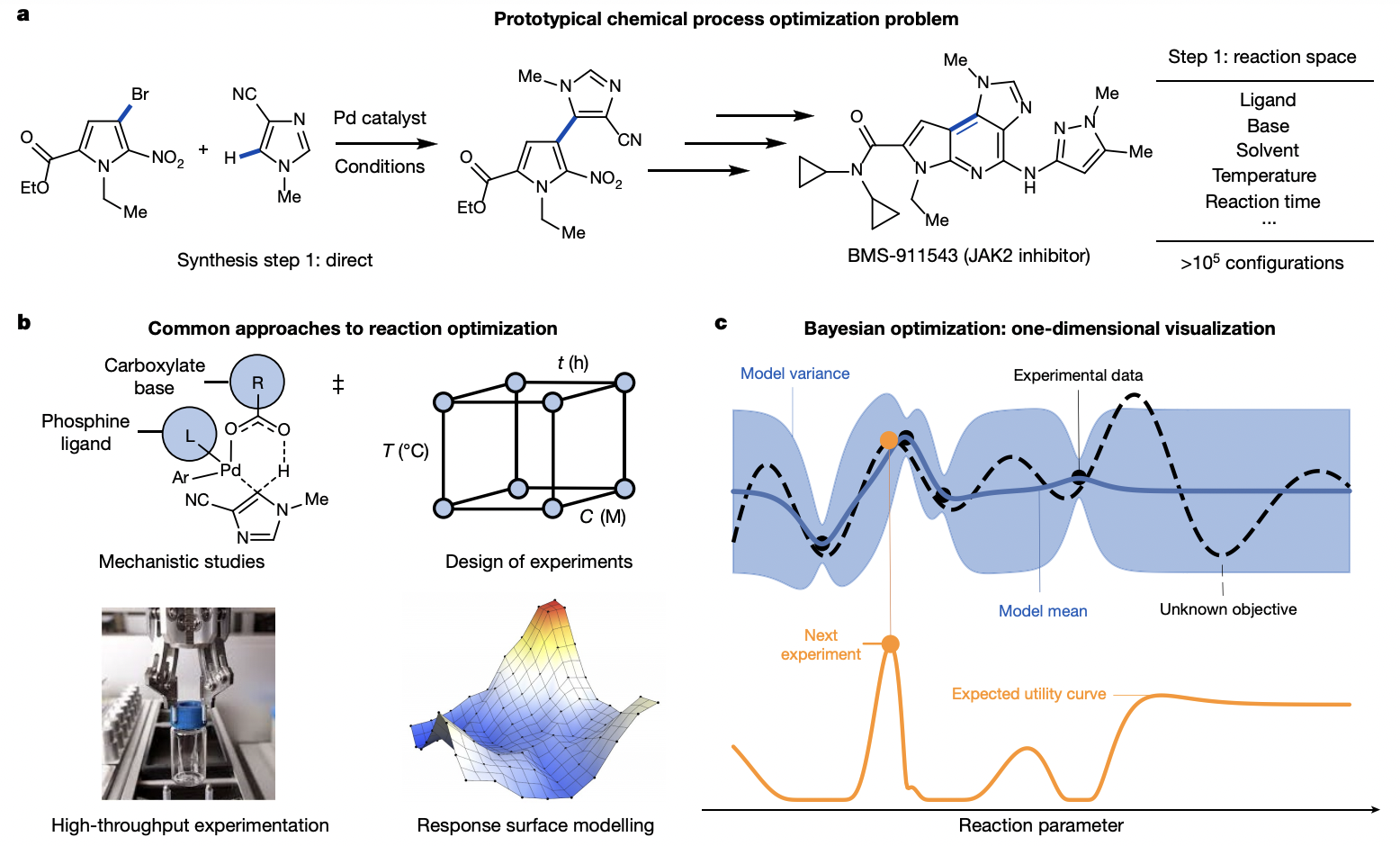
Kariofillis, S. K.; Doyle, A. G. Acc. Chem. Res. 2021, 54, 988-1000.
[DOI: 10.1021/acs.accounts.0c00694] Link PDF
Proppe, A. H.; Li, Y. C.; Aspuru-Guzik, A.; Berlinguette, C. P.; Chang, C. J.; Cogdell, R.; Doyle, A. G.; Flick, J.; Gabor, N. M.; van Grondelle, R.; Hammes-Schiffer, S.; Jaffer, S. A.; Kelley, S. O.; Leclerc, M.; Leo, K.; Mallouk, T. E.; Narang, P.; Schlau-Cohen, G. S.; Scholes, G. D.; Vojvodic, A.; Yam, V. W.; Yang, J. Y.; Sargent, E. H. Nat. Rev. Mater. 2020, 5, 828-846.
[DOI: 10.1038/s41578-020-0222-0] Link PDF
Webb, E. W.; Park, J. B.; Cole, E. L.; Donnelly, D. J.; Bonacorsi, S. J.; Ewing, W. R.; Doyle, A. G. J. Am. Chem. Soc. 2020, 142, 9493-9500.
[DOI: 10.1021/jacs.0c03125] Link PDF
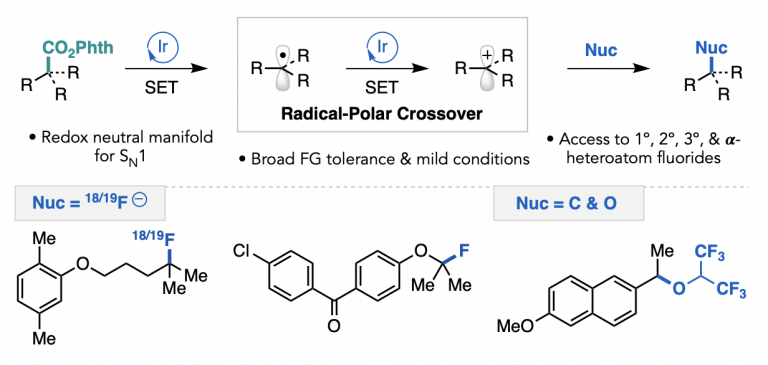
Parasram, M.; Shields, B. J.; Ahmad, O.; Knauber, T.; Doyle, A. G. ACS Catal. 2020, 10, 5821-5827.
[DOI: 10.1021/acscatal.0c01199] Link PDF
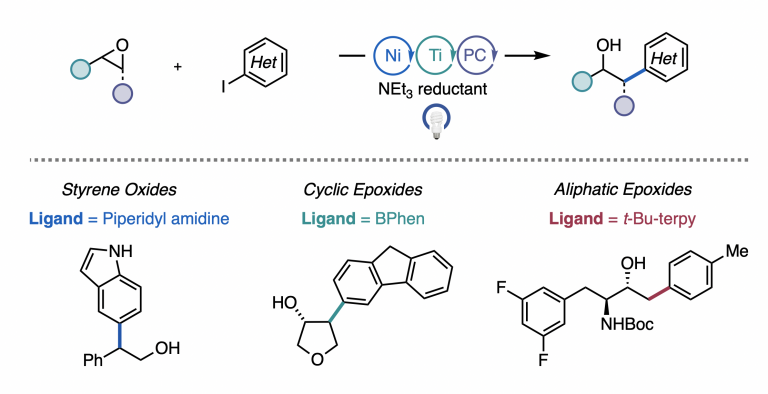
Estrada, J. G.; Williams, W. L.; Ting, S. I.; Doyle, A. G. J. Am. Chem. Soc. 2020, 142, 8928-8937.
[DOI: 10.1021/jacs.0c02237] Link PDF
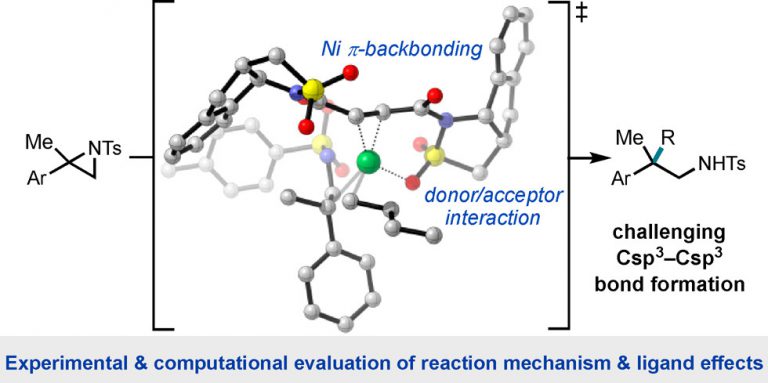
Kariofillis, S. K.; Shields, B. J.; Tekle-Smith, M. A.; Zacuto, M. J.; Doyle, A. G. J. Am. Chem. Soc. 2020, 142, 7683-7689.
[DOI: 10.1021/jacs.0c02805] Link PDF
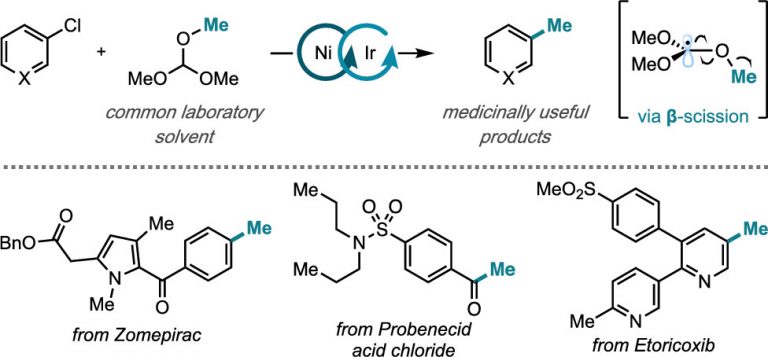
Steiman, T. J.; Liu, J.; Mengiste, A.; Doyle, A. G. J. Am. Chem. Soc. 2020, 142, 7598-7605.
[DOI: 10.1021/jacs.0c01724] Link PDF
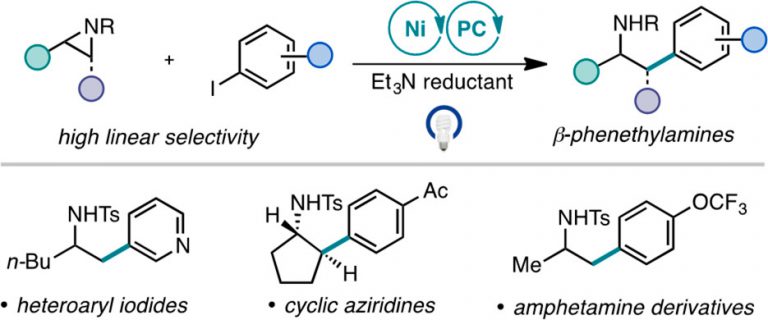
Ting, S. I.; Garakyaraghi, S.; Taliaferro, C. M.; Shields, B. J.; Scholes, G. D.; Castellano, F. N.; Doyle, A. G. J. Am. Chem. Soc. 2020, 142, 5800-5810.
[DOI: 10.1021/jacs.0c00781] Link PDF
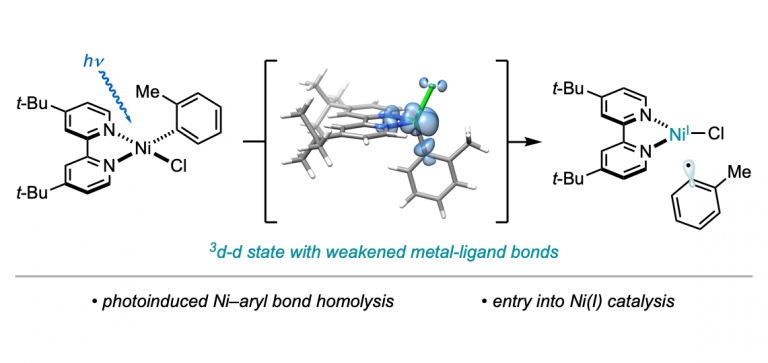
Martinez Alvarado, J. I.; Ertel, A. B.; Stegner, A.; Stache, E.; Doyle, A. G. Org. Lett. 2019, 21, 9940-9944.
[DOI: 10.1021/acs.orglett.9b03871] Link PDF
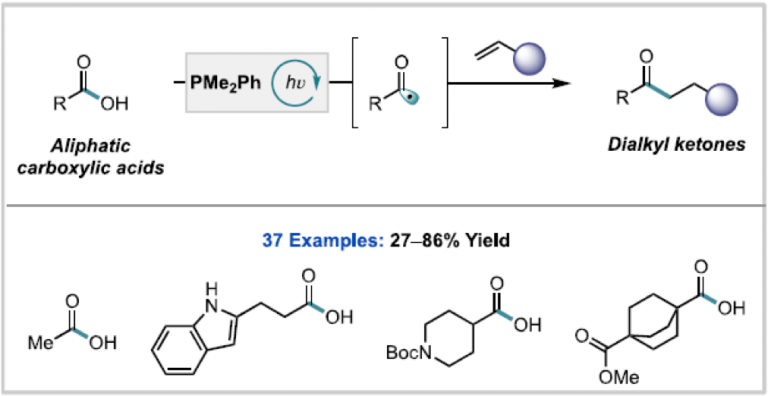
Seff, A.; Zhou, W.; Damani, F.; Doyle, A. G.; Adams, R. P.
[arXiv:1907.08268 [cs.LG]] Link PDF
Estrada, J. G.; Ahneman, D. T.; Sheridan, R. P.; Dreher, S. D.; Doyle, A. G. Science 2018, 362, eaat8763
[DOI: 10.1126/science.aat8763] Link PDF
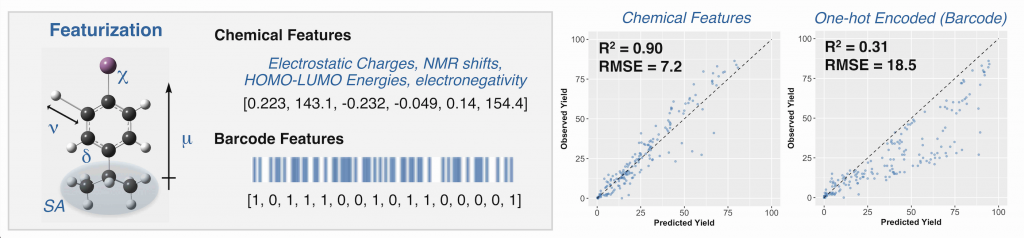
Stache, E. E.; Ertel, A. B.; Rovis, T.; Doyle, A. G. ACS Catal. 2018, 8, 11134-11139.
[DOI: 10.1021/acscatal.8b03592] Link PDF
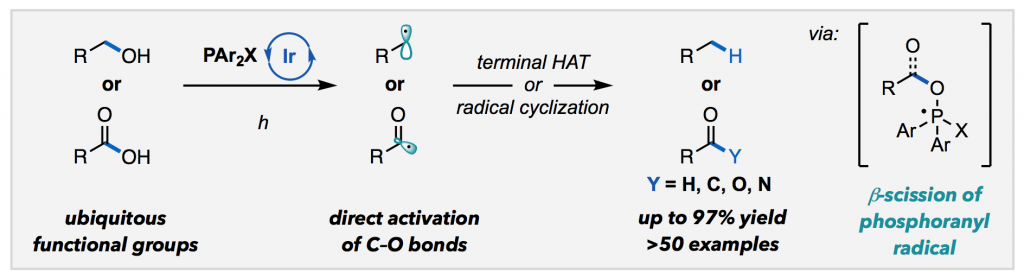
Ackerman, L. K. G.; Martinez Alvarado, J. I.; Doyle, A. G. J. Am. Chem. Soc. 2018, 140, 14059-14063.
[DOI: 10.1021/jacs.8b09191] Link PDF
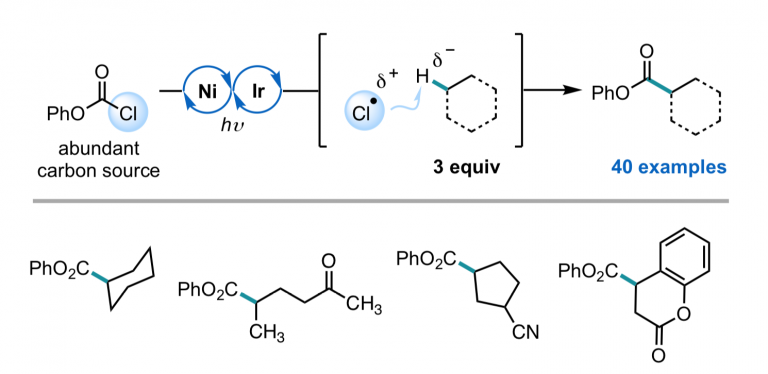
Nielsen, M. K.; Ahneman, D. T.; Riera, O.; Doyle, A. G. J. Am. Chem. Soc. 2018, 140, 5004-5008.
[DOI: 10.1021/jacs.8b01523] Link PDF
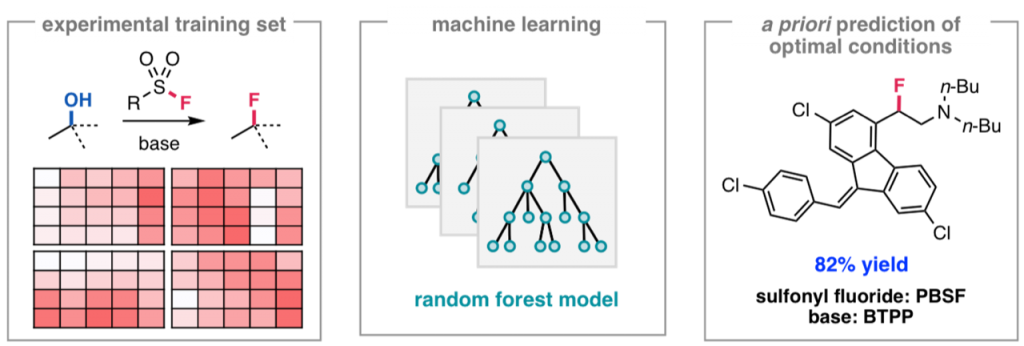
Ahneman, D. T.; Estrada, J. G.; Lin, S.; Dreher, S. D.; Doyle, A. G. Science 2018, 360, 186-190.
[DOI: 10.1126/science.aar5169] Link PDF
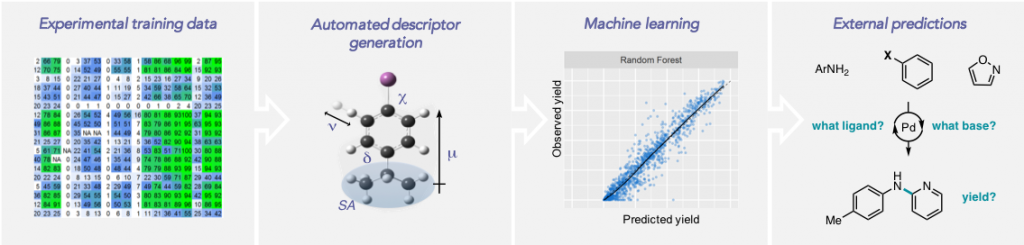
Shields, B. J.; Kudisch, B.; Scholes, G. D.; Doyle, A. G. J. Am. Chem. Soc. 2018, 140, 3035-3039.
[DOI: 10.1021/jacs.7b13281] Link PDF
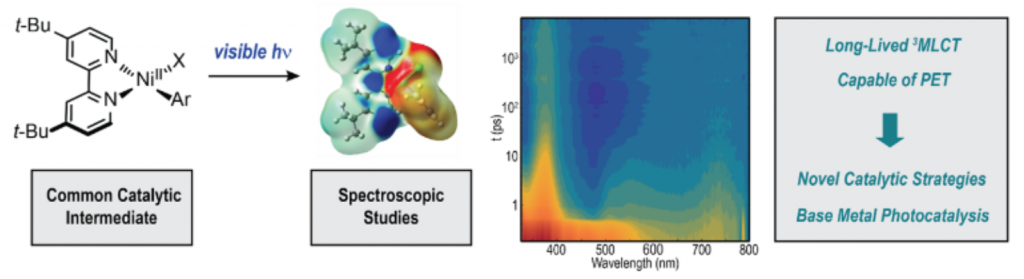
Heinz, C.; Lutz, J. P.; Simmons, E. M.; Miller, M. M.; Ewing, W. R.; Doyle, A. G. J. Am. Chem. Soc. 2018, 140, 2292-2300.
[DOI: 10.1021/jacs.7b12212] Link PDF
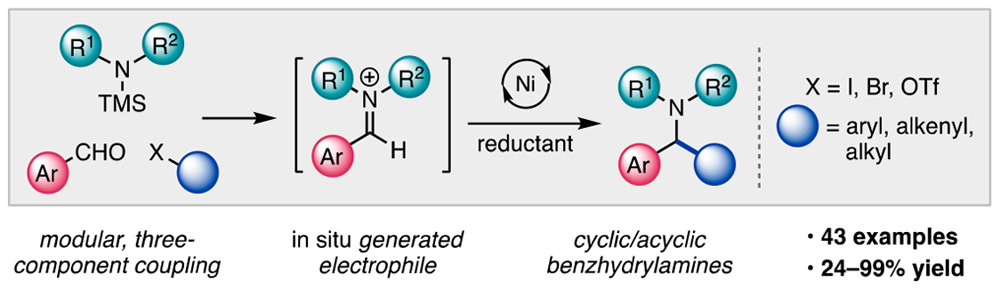
Nielsen, M. K.; Shields, B. J.; Liu, J. Williams, M. J.; Zacuto, M. J; Doyle, A. G. Angew. Chem. Int. Ed. 2017, 56, 7191-7194.
[DOI: 10.1002/ange.201702079] Link PDF

Woods, B. P.; Orlandi, M.; Huang, C.-Y. Sigman, M. H.; Doyle, A. G. J. Am. Chem. Soc. 2017, 139, 5688-5691.
[DOI: 10.1021/jacs.7b03448] Link PDF
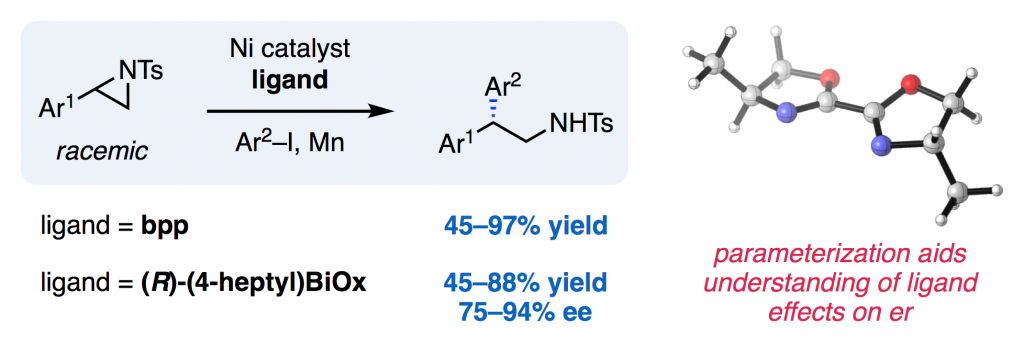
Angew. Chem. Int. Ed. J. Am. Chem. Soc. 2017, 56, 3679-3683.
[DOI:10.1002/anie.201700097] Link PDF
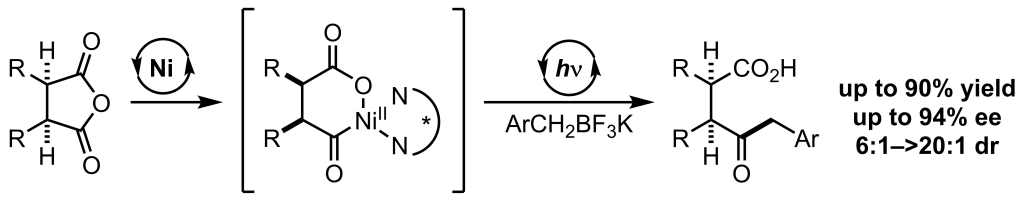
Wu, K.; Doyle, A. G. Nature Chem. 2017, 9, 779-784.
[DOI:10.1038/nchem.2741] Link PDF
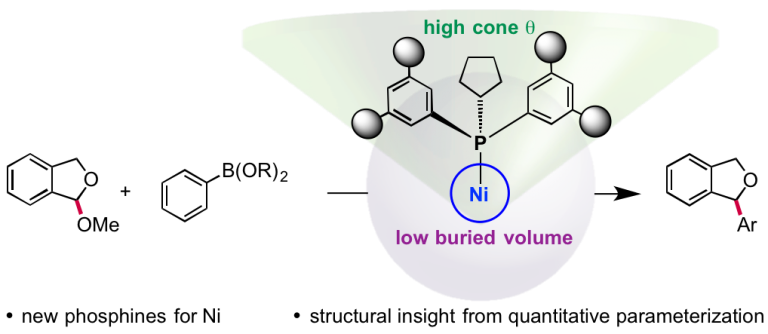
Shields, B. J.; Doyle, A. G. J. Am. Chem. Soc. 2016, 138, 12719−12722.
[DOI: 10.1021/jacs.6b08397] Link PDF
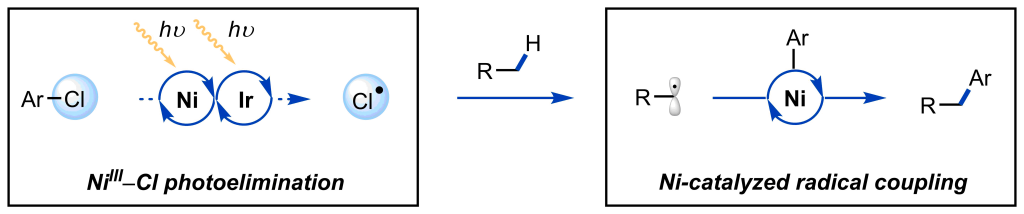
Gray, E. E.; Nielsen, M. K.; Choquette, K. A.; Kalow, J. A.; Graham, T. J. A.; Doyle, A. G. J. Am. Chem. Soc. 2016, 138, 10802−10805.
[DOI: 10.1021/jacs.6b06770] Link PDF
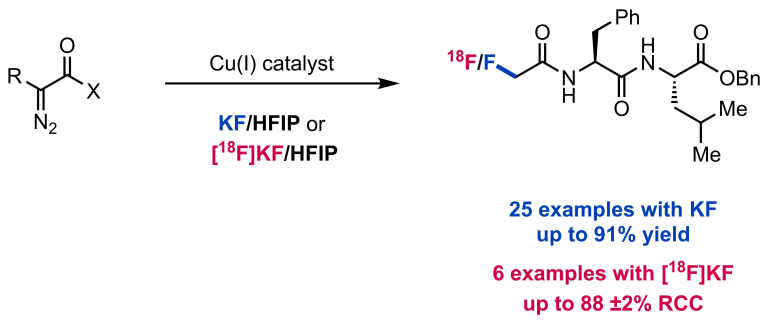
Ahneman, D. T.; Doyle, A. G. Chem. Sci. 2016, 7, 7002-7006.
[DOI:10.1039/C6SC02815B] Link PDF
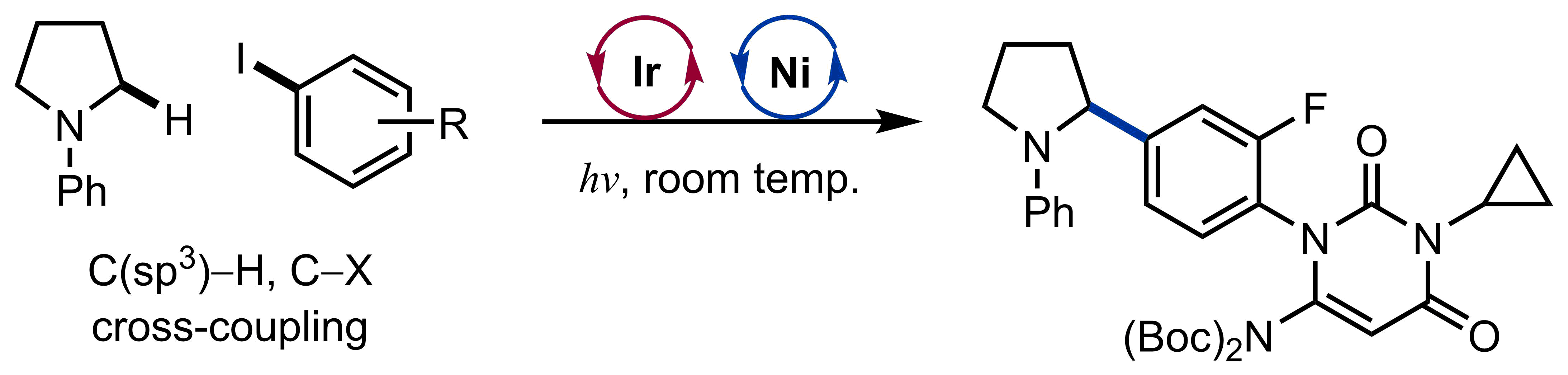
Lutz, J. P.; Chau, S. T.; Doyle, A. G. Chem. Sci. 2016, 7, 4105-4109.
[DOI: 10.1039/C6SC00702C] Link PDF
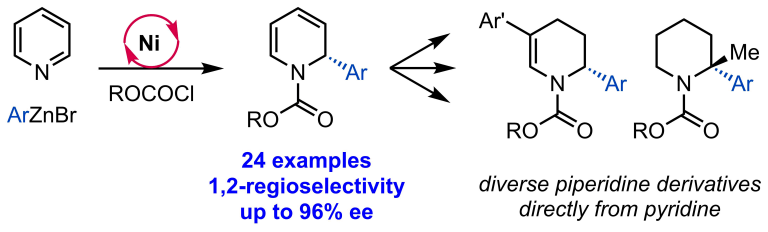
Joe, C. L.; Doyle, A. G. Angew. Chem. Int. Ed. 2016, 55, 4040-4043.
[DOI: 10.1002/anie.201511438] Link PDF
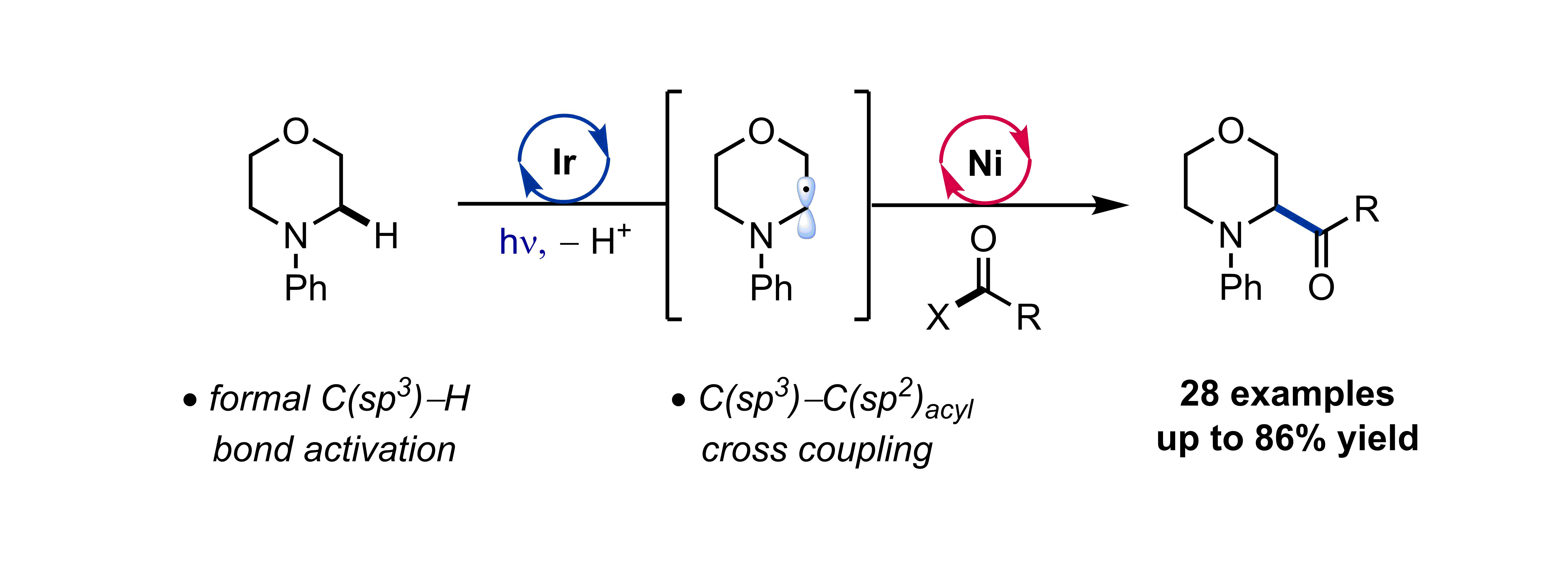
Nielsen, M. K.; Ugaz, C. R.; Li, W.; Doyle, A. G. J. Am. Chem. Soc. 2015, 137, 9571−9574.
[DOI: 10.1021/jacs.5b06307] Link PDF
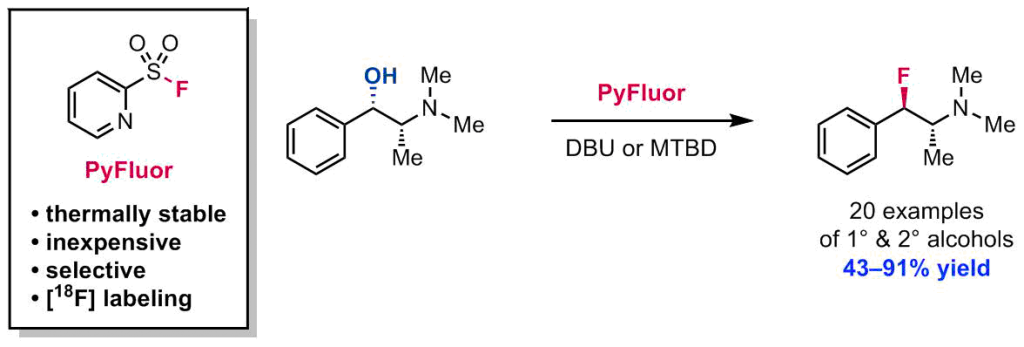
Arendt, K. M.; Doyle, A. G. Angew. Chem. Int. Ed. 2015, 54, 9876-9880.
[DOI: 10.1002/anie.201503936] Link PDF
Huang, C.-Y.; Doyle, A. G. J. Am. Chem. Soc. 2015, 137, 5638−5641.
[DOI: 10.1021/jacs.5b02503] Link PDF
Shields, J. D.; Gray, E. E.; Doyle, A. G. Org. Lett. 2015, 17, 2166−2169.
[DOI: 10.1021/acs.orglett.5b00766] Link PDF
Zuo, Z.; Ahneman, D.; Chu, L.; Terrett, J.; Doyle, A. G.; MacMillan, D. W. C. Science. 2014, 345, 437-440.
[DOI: 10.1126/science.1255525] Link PDF
Huang, C.-Y.; Doyle, A. G. Chem. Rev. 2014, 114, 8153-8198.
[DOI: 10.1021/cr500036t] Link PDF
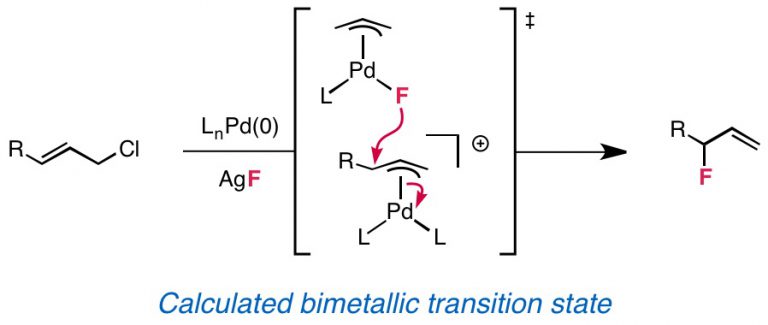
Graham, T. J. A.; Lambert, R. F.; Ploessl, K.; Kung, H. F.; Doyle, A. G. J. Am. Chem. Soc. 2014, 136, 5291-5294.
[DOI: 10.1021/ja5025645] Link PDF
![Enantioselective radiosynthesis of positron emission tomography (PET) tracers containing [18F]fluorohydrins](/wp-content/uploads/2020/07/25-TJG-JACS-2014_TOC.jpg)
Katcher, M. H.; Norrby, P.-O.; Doyle, A. G. Organometallics. 2014, 33, 2121-2133.
[DOI: 10.1021/om401240p] Link PDF
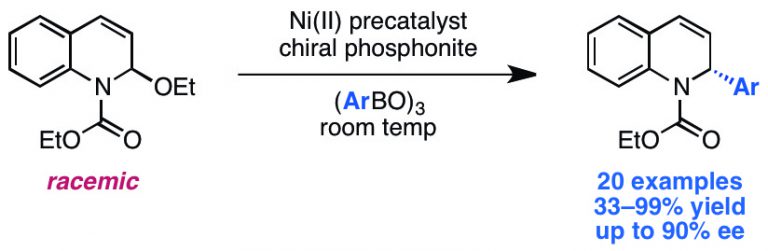
Shields, J. D.; Ahneman, D. T.; Graham, T. J. A.; Doyle, A. G. Org. Lett. 2014, 16, 142-145.
[DOI: 10.1021/ol4031364] Link PDF
Nielsen, D. K.; Huang, C.-Y.; Doyle, A. G. J. Am. Chem. Soc. 2013, 135, 13605–13609.
[DOI: 10.1021/ja407223g] Link PDF
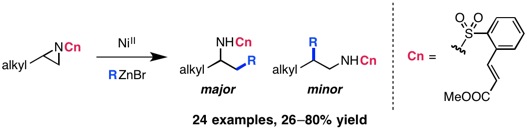
Braun, M.-G.; Doyle, A. G. J. Am. Chem. Soc. 2013, 135, 12990–12993.
[DOI: 10.1021/ja407223g] Link PDF
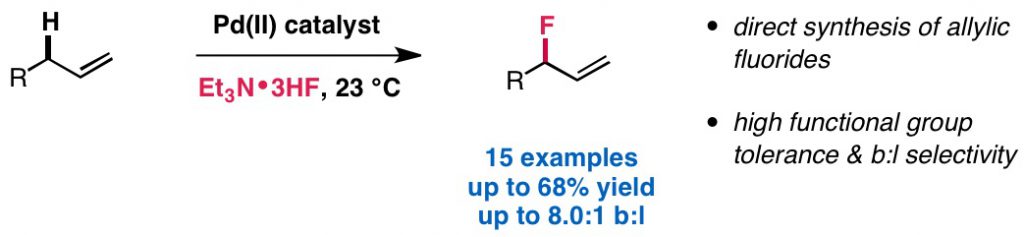
Chau, S. T.; Lutz, J. P.; Wu, K.; Doyle, A. G. Angew. Chem. Int. Ed. 2013, 52, 9153-9156.
[DOI: 10.1002/anie.201303994] Link PDF
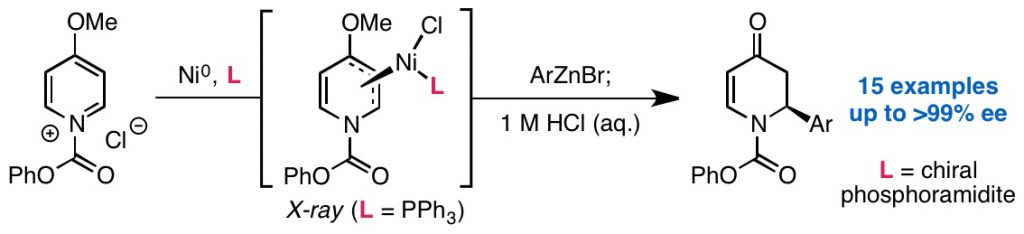
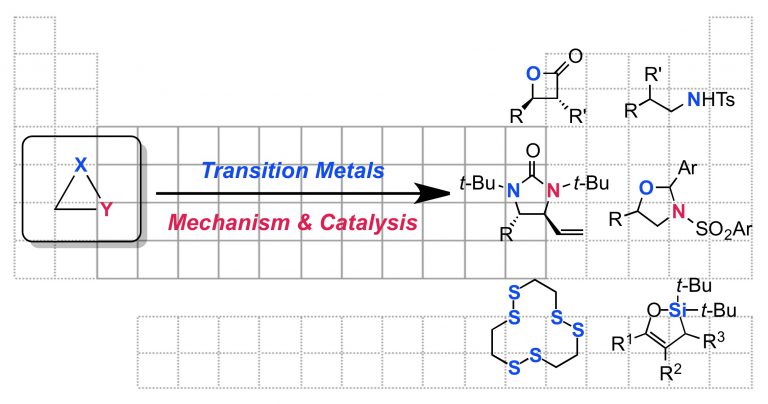
19. Enantioselective fluoride ring opening of aziridines enabled by cooperative Lewis acid catalysis
Kalow, J. A.; Doyle, A. G. Tetrahedron. 2013, 69, 5702-5709.
[DOI: 10.1016/j.tet.2013.01.062] Link PDF
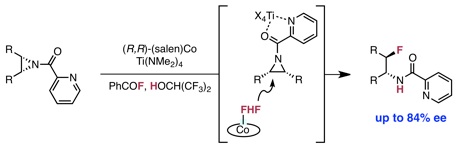
Braun, M.-G.;‡ Katcher, M. H.;‡ Doyle, A. G. Chem. Sci. 2013, 4, 1216–1220. (‡ Equal contribution)
[DOI: 10.1039/C2SC22198E] Link PDF

Sylvester, K. T.;‡ Wu, K.;‡ Doyle, A. G. J. Am. Chem. Soc. 2012, 134, 16967-16970. (‡ Equal contribution)
[DOI: 10.1021/ja3079362] Link PDF

Kalow, J. A.; Schmitt, D. E.; Doyle, A. G. Org. Chem. 2012, 77, 4177–4183.
[DOI: 10.1021/jo300433a] Link PDF
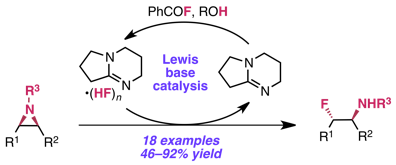
Huang, C.-Y.; Doyle, A. G. J. Am. Chem. Soc. 2012, 134, 9541–9544.
[DOI: 10.1021/ja3013825] Link PDF
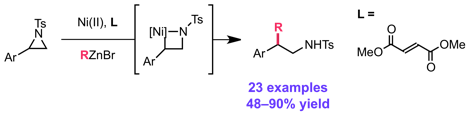
Graham, T. J. A.; Doyle, A. G. Org. Lett. 2012, 14, 1616–1619.
[DOI: 10.1021/ol300364s] Link PDF
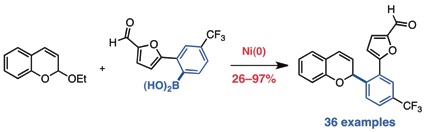
Shaw, T. W.; Kalow, J. A.; Doyle, A. G. Org. Synth. 2012, 89, 9–18.
[DOI: 10.15227/orgsyn.089.0009] Link PDF
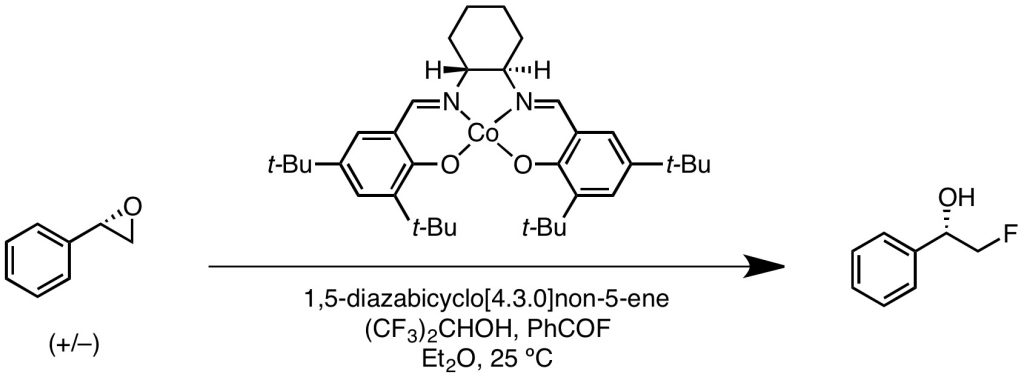
Katcher, M. H.; Sha, A.; Doyle, A. G. J. Am. Chem. Soc. 2011, 133, 15902–15905.
[DOI: 10.1021/ja206960k] Link PDF
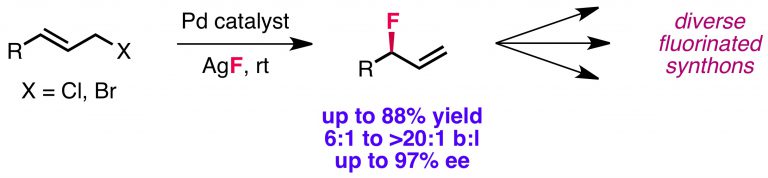
Kalow, J. A.; Doyle, A. G. J. Am. Chem. Soc. 2011, 133, 16001–16012.
[DOI: 10.1021/ja207256s] Link PDF
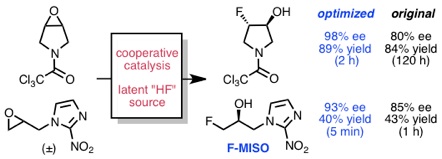
Nielsen, D. K.; Doyle, A. G. Angew. Chem., Int. Ed. 2011, 50, 6056–6059.
[DOI: 10.1002/anie.201101191] Link PDF
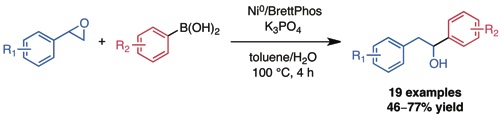
Graham, T. J. A.; Shields, J. D.; Doyle, A. G.Chem. Sci. 2011, 2, 980–984.
[DOI: 10.1039/c1sc00026h] Link PDF
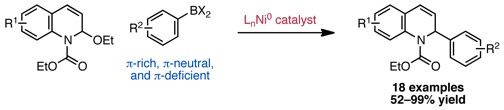
Katcher, M. H.; Doyle, A. G. J. Am. Chem. Soc. 2010, 132, 17402–17404.
[DOI: 10.1021/jacs.0c03125] Link PDF
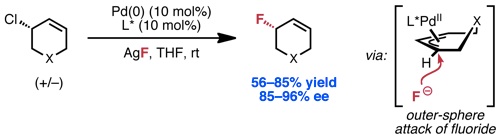
Kalow, J. A.; Doyle, A. G. J. Am. Chem. Soc. 2010, 132, 3268–3269.
[DOI: 10.1021/ja100161d] Link PDF